1. Introduction
Due to the need to reduce environmental emissions, current research is focusing on green or renewable energy alternatives. For this purpose, the use of oils of vegetable origin as a substitute for oil-derived lubricants is an excellent alternative because they have properties such as a high viscosity index, low volatility, high detergency, high dispersibility, biodegradability, low toxicity among others [1-4].
At present, key issues arise from the use of biolubricants produced from vegetable oils. For example, raw material availability which is one of the main obstacles for its industrial development. It is probably just a matter of time, depending on the population of our planet, when neat oil and fat (coming from those species that do not participate in the human nutrition chain) will be allowed in the production of non-food products [5]. At the moment, biolubricants are produced industrially from waste kitchen oils.
However, the direct use of vegetable oils as lubricants is unfavorable because they have low thermo-oxidative stability and decrease in fluidity at low temperatures [1,6]. These deficiencies have been enhanced by the introduction of chemical changes, such as esterification, epoxidation and hydroxylation, where chemically modified products show improvements in oxidation stability and low temperature fluidity [7-10].
It has been observed from several studies that the tribological properties of the lubricants increased when adding some type of substance by which they improve the indexes of viscosity and the anti-wear properties, which influences considerably the quality of a lubricant [10-13].
These substances are known as additives, which traditionally contain sulfur and phosphorus that are highly toxic and are specially formulated for processes where the use of high temperatures is needed. Nanoparticles (NPs) are now being used as an alternative for these substances. The performance of NPs depends to a great extent on their size, geometry and concentration. The lubricating power of the NPs is mainly explained by the formation of an anti-wear film on the friction surface [14-18].
The most used NPs include Cu, CuO, Fe, Ni, TiO2, Fe2O3, FeO, CuO, Cu2O, Al2O3, Mo, Ti, MgO, MoS2 among others which when used in lubricating oils provide a good reduction of friction and improve the anti-wear behavior [15,19-22].
Among these additives, NPsCu have received increased attention because deposited on the friction surface they improve the tribological properties of the lubricant base stocks and show good anti-friction properties and reduce wear [14,23-25]. The use of NPsAl2O3 as an anti-wear additive for lubricant, decreases the coefficient of friction and wear at concentration of 0.1% w/w in oil [26,27].
As NPs are produced commercially, when used as additives in lubricants, different techniques are used, where dispersion is the most prominent and easy to apply; however, due to the no polar nature of oils, this process can be complicated by the formation of aggregations or conglomerates. The latter can be avoided by the use of oleic acid, the use of mechanical agitation, high pressure homogenization, ultrasonic irradiation or ball milling [15,19,28,29].
In this study a biolubricant was made from the mixture of epoxidized sesame (ESO) and transesterified sesame (MES), in order to improve thermo-oxidative stability and low temperature behavior. The nanoparticles were added to the oil mixture to improve lubricant performance.
2. Materials and methods
2.1. Materials
Virgin sesame oil (Sesamum indicum L.) from the Department of Meta, Colombia. Lipid profile in acid percentage: lauric 0.38, myristic 0.18, palmitic 9.0, palmitoleic 0.10, stearic 5.07, oleic 37.69, linoleic 46.59, arachidic 0.46, gamma linolenic <0.05, eicosenoic 0.15, alpha linolenic 0.33. Density: 0.92 g/cm3, viscosity: 34.2 mm2/s, free fatty acids: 0.74%.
For the epoxidation the following reagents were used: 50% H2O2 from the Vikudha® Ltd, acetic acid supplied by Conquimica® and sulphuric acid reagent grade produced by J.T. Baker®. NaHCO3 and pure anhydrous Na2SO4, manufactured by Panreac® Química S.L.U. In the transesterification, anhydrous methanol and sodium methoxide from the Merck® were used.
Dispersing agent nonylphenol ethoxylated 10 moles produced by Dow® Ch. Nanoparticles of Cu and Al2O3 with good anti-wear properties in lubrication have been reported for size ranges between 20-150 nm [30-33], so we chose a diameter of 50 cm which is within the range suitable for lubricating properties. NPsCu (ELt-NCuA®, 50 nm, purity> 99.99%) and NPsAl2O3 (Elt-NAIL50Y®, 50 nm, purity 99.99%) were purchased from Anhui Elite® Industrial Co. Ltd.
For the epoxidation and transeterification reactions, controlled magnetic stirring and heating systems were used. In NP dispersion a TOPT500 ultrasonic homogenizer was used, manufactured by Toption Group® Co., Ltd. and equipped with an ultrasonic generator, a transducer probe of 6 mm and a temperature sensor.
2.2. Methods
2.2.1. Sesame epoxidation (ESO)
The epoxidation of Sesame oil was carried out with the use of peracetic acid. At first, a peracetic acid solution was prepared. The molar ratio between moles of double bonds to CH3COOH to H2O2 was kept as 1:0.5:2, respectively and H2SO4 (4.0 g) was added into the solution. Then, 158.4 g of peracetic acid was added slowly from a dropping funnel to 200 g of sesame oil placed in a round-bottomed flask and a reflux condenser. At the end of the addition step, the mixture was heated to 60 °C and allowed to react for 6 hours. The reaction product was neutralized with 4% NaHCO3 solution and then washed with distilled water [7]. The epoxidized oil was centrifuged to remove the remaining water, and finally to remove moisture anhydrous Na2SO4 was added.
2.2.2. Sesame transesterification (MES)
A solution of sodium methoxide in methanol (2.0 g: 45 g) was added to 200 g of sesame oil preheated to 55 °C. The same epoxidation reactor was used. The temperature was raised up to 60 °C and allowed to react for 90 minutes. The methyl ester was separated from the glycerin and washed three times with deionized water. Then, Na2SO4 was added to dry the product.
2.2.3. Lubricant base stock (ESO/MES)
In order to balance the resistance to oxidation with the behavior at low temperatures, a mixture of 74% of MES and 26% of ESO in volume were prepared.
2.2.4. Dispersion of NPs
The following concentrations were used in % w/w: 0.05, 0.1, 0.15 and 2.0 for both NPsCu and NPsAl2O3. The samples of lubricant base stock with NPs were dispersed in an ultrasonic homogenizer model TOPT 500 of Toption Instrument Co. Ltd (Fig. 1). 60 mL of MES/ESO + 0.5% + NPs were placed in a beaker, it was locked in the dispersion chamber and then a transducer probe (6 mm diameter) was immersed in sample. The equipment was operated at 500 W and 20 kHz. The samples were sonicated for 10 minutes with 3 seconds ON and 2 seconds OFF pulses.
2.3. Methods of testing
2.3.1. Infrared spectroscopy (FTIR)
The parameters employed were reported previously [34], The Nicolet iS10 spectrometer (Thermo Fisher Scientific®) equipped with triglyceride sulphate detector (DTGS) and KBr beam splitter.
2.3.2. Differential scanning calorimetric (DSC)
The analysis of the thermo-oxidative resistance to the MES/ESO lubricant base stock was measured on a DSC Series Q20 calorimeter of TA Instruments®, following reported procedure [35]. With this same equipment the tests of cooling/heating curves were measured according to the parameters described by Delgado et al. [36]; the sample was purged with a nitrogen flow of 20 ml/min and subjected to three temperatures: heating from room temperature to 50 °C held for 5 min., cooling from 50 °C to -60 °C held for 5 min., and finally, heating was carried out from -60 °C to 50 °C.
2.3.3. Scanning electronic microscopy (SEM)
This technique of analysis was used to observe the wear scar morphology of the spheres after tribological test. A JEOL JCM-5000 NeoScope™ was used [36]. The images were taken with acceleration voltage of 15 and 10 kW.
3. Results and analysis
3.1. Characterization of MES/ESO lubricant base stock
Sesame oil is a substance of great interest for the creation of biolubricants due to advantages such as better flowability at low temperatures due to the high concentration of olefinic double bonds from oleic and linoleic acids. Therefore, proposing the epoxidation as an alternative to improve the thermo-oxidative stability showed to be a favorable reaction to achieve this purpose. However, in previous studies, the results indicated that such chemical modification increased the thermo-oxidative stability of the virgin oil but the viscosity of the oil increased considerably from 34.2 mm2/s to 479 mm2/s, which was reflected in the increase of the melting point or cloud point, indicating that the fluidity of the oil decreases at low temperatures.
To improve the fluidity and decrease the viscosity presented in the epoxidized oil, the MES/ESO biolubricant base stock was achieved and its lubricating power was evaluated. Fig. 3 shows the IR spectra of the sesame oils studied. The band at 3007 cm-1 corresponding to the vibration of the =C-H (cis) stretching of olefinic double bonds is observed. The asymmetrical and symmetrical vibrations of the -C-H stretching of the methylene group (CH2) appear at 2915 and 2851 cm-1, respectively. The characteristic band for the stretching of the carbonyl carbon (-C=O) of the ester group of the triglycerides was found at 1740 cm-1. A band at 718 cm-1 is the overlap of CH2 rocking vibration and the vibration of off-plane flexion of cis-disubstituted olefins and is common for the three spectra shown [38,39].

Source: The authors
Figure 3 FT-IR spectrum of virgin sesame oil, ESO and MES/ESO biolubricant base stock
However, there were clear differences between the spectrum represented by MES/ESO and that of ESO. In the first spectrum it is not possible to observe the band 823 cm-1, which indicates the presence of an oxirane group, and this is likely due to the overlap of bands, because this region of the spectrum is quite saturated. The band at 1436 cm-1 only appears in the spectrum of the oil mixture, indicating the presence of the MES product, since it represents the symmetrical and asymmetrical stretching of the carbonyl groups [40].
3.2. Analysis of the thermal behavior for the MES/ESO lubricant base stock
3.2.1. Thermo-oxidative stability
The MES/ESO lubricant base stock influenced the increase in the oxidation start temperature, according to the results shown in Table 1, where the highest TONSET value was 239.79 °C for MES/ESO, with a percentage increase of 28% with respect to virgin oil. The increase in the TONSET values of the modified oils is in agreement with the bibliographical reports, which affirm that epoxidation and transesterification reactions, which chemically modify the structure of the oil, improve the thermo-oxidative properties of vegetable oils [20,38,39,41-43].
3.2.2. Determination of the MES/ESO lubricant base stock
Fig. 4 shows the cooling curve for the MES/ESO lubricant base stock analyzed by DSC. A peak heat flux is observed at -8.62 °C, showing that the thermal behavior of the lubricant base stock during crystallization is simple. This value is a change of phase and being below zero degrees Celsius indicates that the triglycerides of the oil are in the liquid state and without crystals at temperatures that are usually of cooling, and therefore it maintains its oily characteristic and is free from turbidity [44]. For this type of curve, it is usual to see two exothermic peaks, corresponding to the formation of micro-crystals and to the freezing of the sample temperatures [35], however observation was impossible.
3.2.3. Wear prevention
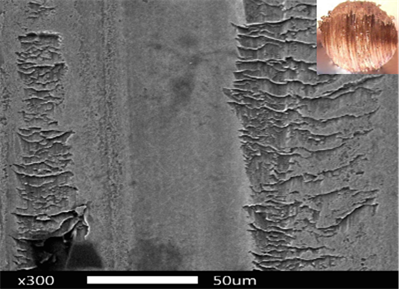
The morphology of the wear scar for MES/ESO, the reference or blank test without NPs is shown in Fig. 5. In this figure it can be seen that the spheres had wear and therefore generated a larger tread size. In the micrograph, vertical roughness and valley formation can be observed, which means that the lubricating power of this mixture is low, causing more friction and therefore more wear. The wear scar diameter (WSD) for the virgin sesame oil reported by Delgado [36] is 2.3 nm and the one found for the MES/ESO lubricant base stock was 1.97 nm, which indicated that the chemical modifications made to sesame oil improved the lubricating power.
3.3. Performance of the NPs in the lubricating capacity
The vegetable oils are known as excellent lubricants because of their molecular structure, due to the formation of intramolecular dipoles, represented by the ester groups from the triglyceride molecules and the hydrophobic tails of the ester which form a protective film that reduces friction and wear [14,27]. Adding metallic NPs improves lubrication capacity. However, metallic NPs have the difficulty of being unstable in a solution. As a consequence of a poor dispersion process or the lack of surface pretreatment of NPs, aggregations, precipitation and oxidation of NPs can be formed [14,19,20], so the successful use of NPs for a biolubricant depends on their dispersion: with a greater dispersion of NPs in the oil the better the lubricant power [14,15,36].
3.3.1. Tribological tests for the lubricant base stock and addition of NPsCu and NPsAl2O3
Fig. 6 shows the relationship between the tread diameter with respect to the percentage concentrations and the type of NPs used. The WSD is higher when the mixture of chemically modified oils without NPs is used. It is clearly observed that the addition of NPs improved the lubricating power of the oil, which is in agreement with what was expected [15,19,27,36,45].
For the NPsCu, increasing the concentration of NPs decreases the WSD, although it is not possible to affirm that there is a significant difference according to the statistic between 0.15% and 0.20%, nor confirm which of the two concentrations is the optimum to use as a lubricant since both have similar WSD values. Between 0.05% and 0.10% there is a difference of approximately 0.1 mm, an acceptable value to assert that at lower concentration of NPsCu the value of WSD is higher. Therefore, it is possible to affirm that if the MES/ESO lubricant base stock had an application of the hydraulic type, low concentrations of NPs could be used, but for an application for gears or bearings it is not possible to determine which concentration is the most indicated since it is necessary to make extreme pressure tests.
With NPsAl2O3, when referring to the MES/ESO lubricant base stock, the WSD decreased considerably, as the addition of NPs improved the lubricating power of the oil. However, unlike the NPsCu, it cannot be stated which of the concentrations used is the best, since there is no marked difference in the values of WSD. With 0.05% of NPsAl2O3 it is already possible to obtain the highest preventive wear.
Fig. 7 shows the SEM images for the MES/ESO lubricant base stock with the NPsCu at different concentrations. When comparing these images with the one of Fig. 5, a noticeable change in the morphology of wear scar is observed, since it is possible to observe a surface without presence of roughness and a more homogeneous one, where there is the presence of vertical lines that indicate the alignment of NPs.

Source: The authors
Figure 7 WSD morphology for the MES/ESO lubricant base stock with NPsCu at a) 0.05% b) 0.10% c) 0.15% d) 0.20%
The white spots observed in the image C indicate the presence of oil or impurities, and with the image D it can be considered that while the surface is smoother and more homogeneous, the wear is less. This is expected, since the studies of NPsCu indicate that these are deposited on the surface of friction, which improves the tribological properties of oils and shows good anti-friction properties and reduced wear [14,24,27,46].
Fig. 8 contains the images for the morphology of WSD for NPsAl2O3 at different evaluated concentrations. The image D corresponding to 0.20% presents greater roughness and impurities than A. However, the appearance of white spots in images B, C and D indicates the presence of agglomerations of NPs, so that at higher concentration of NPsAl2O3 exists lower dispersion, therefore there is an increase of agglomerations [15,26]. This suggests that for this type of NPs it is necessary to use a surface treatment of NPs to investigate a surfactant or dispersing agent when using higher concentrations of 0.10%, but without surfactant a good result can be obtained with concentrations lower than 0.10%.
4. Conclusions
A mixture of chemically modified sesame oils, epoxidation (ESO) and transesterification (MES) was used to make a MES/ESO lubricant base stock, which was characterized by IR, where it was possible to find the bands concerning the expected functional groups; and it was also found by DSC that the lubricant base stock improves the thermo-oxidative capacity with respect to virgin oil, which suggests it as a future basis for the formulation of a biolubricant, since the cooling and heating curves show that crystallization and melting temperature of the oil ratio is -8 °C, indicating that at low temperatures it maintains fluidity.
Tribological trials showed that the addition of both copper and alumina NPs improves the lubricating capacity of the chemically modified sesame oil blend. With NPsCu, the higher the concentration the less the wear scar, where the concentration of 0.15-0.2% is, as stated by the SEM, presents the best results. When NPsAl2O3 was used, the best results were obtained with the lowest concentration (0.05%), as the increase in concentration of NPs resulted in a higher formation of agglomerates, which prevented a better dispersion.