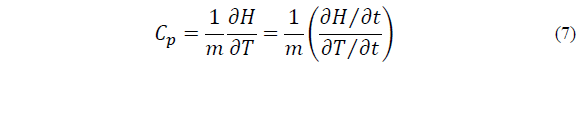1. Introduction
Ice cream is a pasteurized, homogenized, frozen food product characterized by a complex matrix that contains proteins, fat, air, minerals, additives, and sweeteners [1]. Among the many available sweeteners, the most commonly used in the production of ice cream are sucrose, glucose, lactose, and fructose. These sweeteners are intended to provide energy and a sweet taste to the product and to reduce the freezing point of the mixture; however, they also act as antifreeze agents and increase the nonfat solids content of the mixture [2]. Because of the increasing number of people with metabolic disorders, there is a corresponding increase in the demand for low-calorie foods, which is the motivation for this study of alternatives in ice cream formulation. Stevia is a sweetener that has gained increasing interest recently because of its high sweetening power (SP) [3].
Moreover, consumer demand has forced the industry to offer food with better nutritional and sensory quality through innovative ingredients [4] Whey (a byproduct of cheese manufacturing), whose primary components are lactose and nitrogenous protein compounds [5,6], can be used as a substitute for nonfat solids in the manufacturing of ice cream. Whey improves the sensory characteristics, such as a colder feeling, and the textural characteristics, such as the creaminess and the resistance to melting. Whey proteins also improve flavor absorption because they have a high molecular weight, increase the consistency of the ice cream by promoting the formation of smaller ice crystals, and improve the agglomeration of fat globules [2,7].
In the preparation of ice cream, in addition to composition, the thermal properties are important. These properties are essential to estimate freezing times and, of particular importance, to determine the quality and stability of the ice cream as affected by processing and storage temperatures [8].
Most studies aim to replace fats and sugars that have high caloric values with substitutes that do not add many calories to the product [9,10]. Therefore, new ingredients or changes in formulation, such as the addition of whey and gums, have been proposed to improve sensory quality and increase creaminess and melt stability [6,11,12]. These studies were limited to investigating the effects of formulation on quality. We have not yet found any studies on the relation between the quality parameters and the thermal properties of the mixture.
The aim of this study was to assess the effects of replacing the sucrose and the nonfat solids with sweeteners and whey powder, respectively, on the thermophysical properties and the quality parameters of dairy ice cream.
2. Materials and methods
2.1. Ingredients
Homogenized (UHT) whole milk (ALQUERÍA S.A, Colombia), nonfat dry milk powder (COLANTA, Colombia), homogenized and pasteurized milk cream (ALQUERÍA S.A., Colombia), whey powder (TECNAS S.A., Colombia), sucrose (MANUELITA S.A., Colombia), fructose (Laboratorio San Jorge Ltda., Colombia), stevia (Shanghai Tianjia Biochemical Co. Ltda., China), emulsifier-stabilizer (CIMPA S.A., Colombia), and vanilla essence (Royal Alimentos Ltda, Colombia) were the ingredients used for the ice cream formulation. Table 1 shows the formulation and the fat and nonfat solids content for each test sample.
2.2. Ice cream preparation
One liter of the mixture was prepared for each test. The liquid ingredients were mixed and heated to 50°C. The combined dry ingredients were added and mixed using a stirrer (DLS model, Velp Scientifica, Europe) at 800-1000 rpm. The mixture was pasteurized at 80°C for 30 s, cooled to 4°C and matured at 4ºC for 24 hours. Finally, the mixture was frozen at -22°C for 50 min using an ice cream maker (Princess, Model 282600, Germany) at 1800 rpm and stored at -17°C [13]. The prepared ice cream met the minimum requirements of 26 % nonfat solids (NFS) and 10 % fat solids (FS), according to the Colombian Technical Standard [14].
2.3. Experimental design and statistical analysis
An extreme vertices mixture design method for 4 components (whey powder (WP), sucrose (SU), fructose (FR), and stevia (ST)) was used to determine the optimal proportions of the components (g/100g). The experimental design is shown in Table 2.
Equations (1) to (6) show the linear restrictions for each component with respect to the SP and the quantity of NFS that they contribute to the mixture. The response variables in the ice cream mixture after maturation were the thermal properties (specific heat capacity, thermal diffusivity and thermal conductivity), the physical properties (density and consistency coefficient), and the parameters of ice cream quality (overrun, time until the first drop falls and melting time). An analysis of variance (ANOVA) was performed to find the optimal treatment, maximizing the quality parameters. The statistical software Minitab (version 17.1.0, Minitab, Inc.) was used.
2.3.1. General restrictions
ICONTEC [14] requires ice cream to have a minimum of 26 % NFS and 10 % FS. With this restriction, and including all ingredients, a system of equations was developed. The formulation with the least sucrose content was chosen from among the possible combinations, shown in Eq. (1) as follows:
Where WP, SU, FR, and ST are the concentrations of the sweeteners (g/100 g).
2.3.2. Restrictions on the NFS
The restrictions on the NFS based on sucrose substitution are described by Eqs. (2) to (6) as follows:
2.3.3. Restriction on the SP
The SP restriction (Eq. (6)) determines the quantity of sweetener that is added to the mixture, which should not be greater than 12.2 g/100 g.
2.4. Thermal properties
2.4.1. Specific heat capacity (Cp)
The C p (J kg-1 K-1) of the mixture was determined using a differential scanning calorimeter (DSC) (Mettler Toledo). Approximately 20 mg of the sample was added to an aluminum crucible. The following protocol was then performed: the sample was a) cooled from 25°C to -80°C at 10°C/min, b) maintained at -80°C for 10 min to promote icing, and c) heated from -80°C to 80°C at 10°C/min [9]. The C p was determined by the relation between the derivatives of enthalpy (H, mJ) and temperature (T, K) with respect to time (t, s), according to Eq. (7). The C p was calculated as the mean of the values obtained during the freezing stage (the linear section of the thermogram H vs. t).
2.4.2. Thermal conductivity (k)
The k (W m-1 K-1) of the sample was determined by applying Eq. (8) to the data from the melting curve obtained while measuring the C p , where S is the slope of the linear section of the ice-melting peak, h is the height of the aluminum crucible (1.6mm) and A is the radial area of the crucible (28.27mm2) [15] as follows:
2.5. Physical properties
2.5.1. Density (ρ)
The gravimetric-volumetric method, which relates the mass of the ice cream mixture at 4°C to the occupied volume of the sample, was used to calculate ρ (Eq. 10), where m is the mass of the mixture (kg), and V is the volume occupied by the sample (m3), as follows:
2.5.2. Consistency coefficient (K)
A rotational viscometer (Brookfield LVDV-III-U), coupled to a cooling recirculating bath at a constant temperature (4°C), was used to determine the rheological behavior of the dairy ice cream sample after maturation. The consistency coefficient (K, Pa·sn) and the flow behavior index (n) were calculated from the shear stress (σ, Pa·s) and the shear rate (γ, s-1), using Eq. (11) [9] as follows:
2.6. Quality parameters
2.6.1. Overrun (O)
Overrun (%) is defined as the volume change resulting from the aeration, i.e., caused by the capacity of air incorporated into the ice cream mixture, and described by Eq. (12) as follows [17]:
Where V m is the volume of the mixture and V i is the volume of the ice cream.
2.6.2. First drop time (D) and melting time (M)
Approximately 50 g of ice cream was placed in a mesh (56 holes/cm2) and held at room temperature, controlled at 24.7±0.96°C.
Table 3 Thermophysical properties and quality parameters

C p : Specific heat capacity, α: Thermal diffusivity, k: Thermal conductivity, ρ: Density, K: Consistency coefficient, n: flow behavior index (dimensionless), O: Overrun, D: First drop time, M: Melting time.
Source: The Authors
The first drop time (D) was determined and the weight of the melted ice cream was recorded as a function of time. The melting time (M) was defined as the time required to melt 30 g of the product [18].
2.6.3. Sensory analysis
Descriptive statistics were applied to assess the difference between the optimal formulation and a commercial ice cream sample. The assessment was performed with 44 untrained evaluators. All samples were served in cylindrical shapes 3 cm high with 2 cm diameters in 30 ml plastic containers with lids [19].
3. Results and discussion
The results for each response variable, according to the experimental design, are shown in Table 3.
3.1. Effects of the mixture ingredients on the response variables
The contour graphs of the proportions of the ingredients WP, SU, and FR, considering the restrictions related to NFS for each experimental response are shown in Figs. 1 to 8. The vertices correspond to each of the pure components in the mixture, i.e., the vertex of WP represents 12.2 % WP and 0 % sweetener). The stevia (ST) was omitted because the mass ratio of ST with respect to the others is very low, and the effect of ST on the response variables is reflected in the ratio of WP used to meet the required NFS level.
3.1.1. Specific heat capacity
The results for C p are shown in Fig. 1. The value of C p for the mixture decreases as the proportions of SU and FR increase and the amount of WP decreases; values from 1646 to 1937 J/kg K were measured.
Agrawal, et al. [20] calculated values between 2516 J/kg K and 2542 J/kg K from the C p of each ingredient for ice cream with ginger juice. The composition in their study was 26 % NFS and 12 % FS (the C p of fat is 2.09 kJ/kg K and the C p of NFS is 1.93 kJ/kgK). It is possible that the differences between values are from the methods used for the measurements of C p (indirect, depending on the composition vs. direct, using DSC). In these analyses, it is considered that the air incorporated in the ice cream mixture has a negligible effect on the thermal and physical properties [21]. In general, a low C p indicates a short ice cream hardening time [22].

Source: The Authors
Figure 1 Contour graph for specific heat capacity (J kg-1 K-1) in function of the formulation
3.1.2. Thermal conductivity
The results of the measurements of k are shown in Fig. 2. The measured k for the ice cream mixture varied between 0.237 W/m K and 0.374 W/m K.
Cogné, et al. [21] mentioned that the ice cream mixture can be represented as a material with two phases, a solid phase (ice crystals) dispersed in a continuous fluid phase composed of dry matter and unfrozen water at -20°C. These authors reported k values from 0.466 to 1.51 W/m K in commercial ice cream with 9.3 % FS and 31.83 % NFS. The difference in k is probably from the state of the sample because the k of the liquid is lower than the k of the solid, e.g, k water is 0.58 W/m K and k ice is 2.18 W/mK. Additionally, the high proportion of NFS in the composition of the food increases the thermal conductivity. Sudhir and Ashis [23] reported k of 0.543 to 0.580 W/m K for ice cream with 27.95 % NFS and 13 % FS and noted that the air (k air is 0.02 W/m°C) inside the food matrix has a negative effect on the thermal conductivity; however, this influence is not addressed in current literature. Additionally, the properties of a food depend primarily on its composition. A lower C p implies that less heat must be eliminated from the material, whereas a lower k indicates that the rate at which heat is eliminated is also lower [24].
3.1.3. Thermal diffusivity
The results in Fig. 3 show the thermal diffusivity varying from 0.1259 to 0.1774 mm2/s. These results are consistent with the results obtained by Ben-Yoseph and Hartel [25] in vanilla ice cream with 12 % FS and 27.5 % NFS, who reported values of 0.01667 mm2/s and 0.15 mm2/s at -5°C and -20°C, respectively.
The value of α indicates how quickly heat can be eliminated during ice cream hardening under storage. The value of α is primarily determined by the composition of the product and the quantity of air inside the mixture. This property is not fully understood in ice cream. It is likely that α is also influenced by the overrun, the cell size, the air distribution, the ice quantity and the sizes of the ice crystals. Increasing the quantity and the dispersion of the air cells and the ice crystals should reduce the thermal diffusivity, resulting in slower melting, i.e., a higher melting time [2].
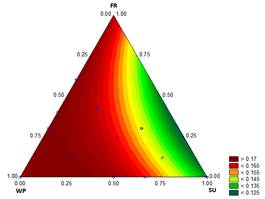
Source: The Authors
Figure 3 Contour graph for thermal diffusivity (mm2 s-1) in function of the formulation
3.1.4. Density
The results in Fig. 4 show the influence of the ingredients on the density (ρ) of the ice cream mixture.
The density changes depending on the composition, i.e., ρ increases as the amount of NFS increases and as the fat content decreases [2]. The density values ranged from 1118 to 1143 kg/m3. As the WP content increased, the density of the mixture decreased. This trend is related to the emulsifying and foaming properties of WP. It is also related to the density of each of the components, i.e., ρ of FR and SU are 1690 kg/m3 and 1590 kg/m3, respectively, whereas ρ of WP is from 0.7 to 0.85 kg/m3 [26]. Warren and Hartel [27] reported density values for an ice cream mixture from 1070 to 1160 kg/m3. Those values are comparable to the density values found in this study.
3.1.5. Rheological properties
The results for the consistency coefficient (K) of the ice cream mixture are shown in Fig. 5. This variable is a key attribute

Source : The Authors
Figure 5 Contour graph for consistency coefficient (Pasn) in function of the formulation
because it influences the body and texture of the final product [28]. Values of K equal to 2.022 Pa·sn and to 11.267 Pa·sn were obtained for formulations containing 12.2 % SU and 12.1782 % WP with 0.0218 % ST, respectively. Other studies have reported similar K for ice cream mixtures [29-31].
Dertli, et al. [29] found consistency coefficients of 0.03 Pa·sn and 24.07 Pa·sn for ice cream mixtures without and with fermentation with Streptococcus thermophilus strains. In the case of the fermented ice cream, the consistency coefficient was higher because of the exopolysaccharides. A higher proportion of WP is positively related to K in the matured dairy ice cream mixture.
The flow behavior index (n) is between 0.419 and 0.71, which indicates that the ice cream mixtures are non-Newtonian fluids that exhibit a pseudoplastic behavior (Table 3). Non-Newtonian behavior is generally associated with intermolecular forces and with the effect of the addition of WP on the protein molecule sizes [32]. The n values obtained are consistent with published studies. Toker, et al. [31] studied the effect of processing temperatures in the range of 5 to 35°C on the K of the ice cream mixture (7.036 to 12.883Pa·sn) and on n (0.328 to 0.360). Also Dogan, et al. [30] analyzed the variations in K (0.277 to 74.6Pa·sn) and in n (0.07 to 0.527) caused by the effect of the xanthan gum concentration in ice cream mixtures.
3.1.6. Overrun
The results of the overrun measurements are shown in Fig. 6. The overrun was between 48 % (treatment with 12.2 % SU) and 126 % (treatments with 12.1782 % WP - 0.0218 % ST). Muse and Hartel [33] reported values from 40 to 70 % overrun for ice cream stored in a batch freezer. The overrun results were good because of the high quantity of air incorporated into the mixture, i.e., the result was a soft ice cream with small crystals that is more resistant to recrystallization during storage [34].
The overrun increased as the WP was increased, and decreased as the proportion of SU increased. This finding could be related to the viscosity and to the difficulty of trapping air in less consistent mixtures (low K). Goff, et al. [35] indicated that whey proteins possess the ability to stabilize emulsions, reducing the interfacial tension and forming a membrane that allows partial coalescence, and therefore improving the binding between fat globules. In addition, stable foams can be formed when heat and the casein micelles interact, resulting in shear stress. Therefore, the use of WP improves the ice cream properties, giving a mild sweetness, increasing the nutritional value and providing good emulsifying properties, structure and texture. This is consistent with the higher values of overrun that are a direct function of the proportion of WP added to the mixture.
3.1.7. First drop time and melting time
The results for the D and the M of the ice cream samples are shown in Figs. 7 and 8, respectively. These characteristics are particularly important to the consumer. Values of D and M for the treatment with sucrose at 12.2 % were 8.11 min and 19.5 min, respectively, while for the treatment with 12.1782 % WP - 0.0218 % ST, the D and M values were 13.76 min and 33.83 min, respectively.
Bahram-Parvar, et al. [36] reported melting times between 37.40 min and 50.80 min when adding gums to the mixture. However, the range of melting rates is consistent with the study of Karaca, et al. [37], who reported a melting rate of 1.17 to 1.91 g/min for ice creams that contain different fat substitutes, values slightly lower than those found in the present study (1.54 g/min and 3.69 g/min). The melting resistance in samples with high proportions of WP is from the increased viscosity of the mixture. In addition, when the overrun is high, the melting time is also high from the effect of the air bubbles in the mixture [27].
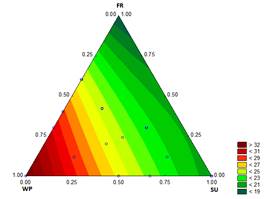
Source: The Authors
Figure 8 Contour graph for melting time [min (30 g)-1] in function of the formulation
The behavior of the mixture can be associated with the rheological properties of the frozen phase of the ice cream. Ice crystals melt and the water diffuses in the frozen phase. The formulations of ice cream with high consistency coefficients and high viscosities have high melting resistance, i.e., the ice cream melts slowly [33].
3.2. Statistical analysis
Polynomial models describe the correlation between the responses and the variables, as shown in Table 4. The coefficients of determination (R2 and adjusted R2) indicate that the selected models have a good fit of the data. A linear model was chosen for C p and k, quadratic models were adjusted for α, K, O and D and a cubic model was adjusted for the variables ρ and M. In the regression models, only the sources of variation with p ≤ 0.05 were included. The positive sign of a coefficient in the adjusted model indicates the magnitude of an increase in the variable, while a negative sign shows a decrease.
3.3. Optimization of the formulations
The optimal proportions of each ingredient to satisfy the quality parameters shown in Table 5 were determined. Consumers prefer ice cream with a low melting rate, i.e., both D and the M should be high.
The industry prefers to maximize the overrun. Hence, these three quality parameters were the response variables to maximize, giving to all of them the same weighing. The optimal proportions were determined to be 12.1782 % WP, 0 % SU, 0 % FR, and 0.0218 % ST. This result indicates that sucrose can be completely substituted by sweeteners with low-caloric value and high sweetening power by taking advantage of WP to produce a dairy ice cream with vanilla flavor that has very good quality features for high consumer acceptability. This promising result can be important for people that cannot consume ice cream because of its high sugar content.
Table 4 Mathematical models for determination thermophysical properties and quality parameters

C p : Specific heat capacity (J kg-1 K-1), α: Thermal diffusivity (mm2 s-1), k: Thermal conductivity (W m-1 K-1), ρ: Density (kg m-3), K: Consistency coefficient (Pa.sn), O: Overrun (%), D: First drop time (min), M: Melting time (min/30g), WP: Whey powder (g/12.2 g sweetener), SU: Sucrose (g/12.2 g sweetener), FR: Fructose (g/12.2 g sweetener) y ST: Stevia (g/12.2 g sweetener).
Source: The Authors
A sample of ice cream with the optimized formulation was prepared to evaluate it and validate the models. The properties of the sample are shown in Table 6. The results presented in Table 6 show moderate errors in most cases.
3.4. Correlation of the response variables
A nonlinear regression was used to mathematically describe the relation that exists among the quality parameters as a function of the thermal properties of the dairy ice cream. With these correlations, the ice cream behavior can be predicted, depending on the thermal properties of the ice cream mixture. The mathematic correlations and the coefficients of determination R2 and adjusted R2 are listed in Table 7. The properties of the sample are shown in Table 8. Small differences were found between the observed and the predicted values, verifying the optimization process.
Table 7 Correlations for determining quality parameters depending on the thermal properties

O: Overrun (%), D: First drop time (min), M: Melting time (min/30g), Cp: Specific heat capacity (J kg-1 K-1), α: Thermal diffusivity (mm2 s-1), k: Thermal conductivity (W m-1 K-1), ρ: Density (kg m-3).
Source: The Authors
3.5. Sensory analysis
The results of the sensory evaluation of pair-wise comparisons to differentiate between the optimal experimental sample and the commercial sample showed no significant difference (p ≤ 0.05), with a proportion of the total population of evaluators able to differentiate between both samples p d of 50 % and a type II error β of 0.01 No differences between the optimal formulation and the commercial sample were found by consumers, verifying that the optimized formulation shows similar sensory properties to the commercial product [19, 38].
4. Conclusions
The formulation of the mixture has influenced the thermophysical properties and quality parameters of a dairy ice cream with vanilla flavor. Whey powder and stevia were the most influencing ingredients. An extreme vertices mixture design was applied to find the formulation with the greatest overrun, the highest first drop time, and the highest melting time to satisfy an industrial need and the consumers’ requirements. The optimal formulation replaced all the sucrose with stevia and added whey powder. This formulation exhibited sensory characteristics that were not significantly different from a commercial sample. Mathematical correlations were developed to predict the parameters of ice cream quality as a function of the thermal properties of the ice cream mixture.