1. Introduction
The huge enrichment of nutrients such as nitrogen and phosphates or the eutrophication of water bodies like lagoons have grown quickly as a result of human being activities. The effects of eutrophication that appear through the massive growing of phytoplankton, algae and macrophyte biomass, proliferation of toxic algae, the increment in water turbidity; the pronounced growth of organic waste matter originated by dead vegetation, the development of hypoxic and anoxic conditions, the decreased diversity of species, the variation of dominant biota and the increased sedimentation level helps the process of blockage and then reduces the longevity of lagoons [1, 2].
Low concentrations of nutriments will determine oligotrophic environments known as “water of good quality”. Its main characteristic is that these waters are of low productivity and high transparency. On the other hand, high amount of nutrients give room to eutrophics environments named “water of low quality” in which the high productivity generates a reduced transparency of the water [3.4].
In a lagoon, water quality monitoring and management are essential as to determine its usage [4]. Among the main factors to consider are the concentration of phosphorus, nitrogen, dissolved oxygen and chlorophyll-a, as well as turbidity, the chemistry and biochemistry demand of oxygen, all of them indicative of eutrophic level of a water body.
Hydrologically, Tecocomulco´s lagoon, it is located in the region called Pánuco River corresponding to the Gulf of Mexico, the basin is considered one of the most important in Mexico, ranking fourth in the national level due to its surface and fifth due to the volume of its runoff. The objective of this work has been to evaluate the trophic status of the Tecocomulco´s lagoon, Hidalgo, Mexico.
2. Materials and methods
2.1. Study area in research
Tecocomulco´s lagoon in Hidalgo Mexico (Fig.1) is located in the oriental site of the volcanic Trans-Mexican belt. It belongs to the Tecocomulco sub-basin, located in the northeast part of the Mexico valley basin. It is delimited by the geographic coordinates 19° 42´13.7” and 19° 59´ 30” north latitude, and 98° 11´ 46.2” and 98° 27´ 30” west longitude; the sea level is 2514 (MSL) average altitude, and depending on the seasonal precipitation its average is 500 to 600 mm. From April to October the period of maximum rains is recorded and between November and March the driest periods. The lagoon has a lengthen shape oriented from northeast to southeast. The average tidal is from 70 cm deep to reach 2 meters depth [5]. The weather in the region is warm climate sub-humid with an average temperature between 8°C to 13°C [6].
2.2. Sampling
Five sample places were distributed based on accessibility in those areas (Table 1 and Fig. 1). The samples were taken in four seasons of the year: March, June, September and December 2009, with the goal of knowing the physic biochemistry variations of water between dry season and rainy season.
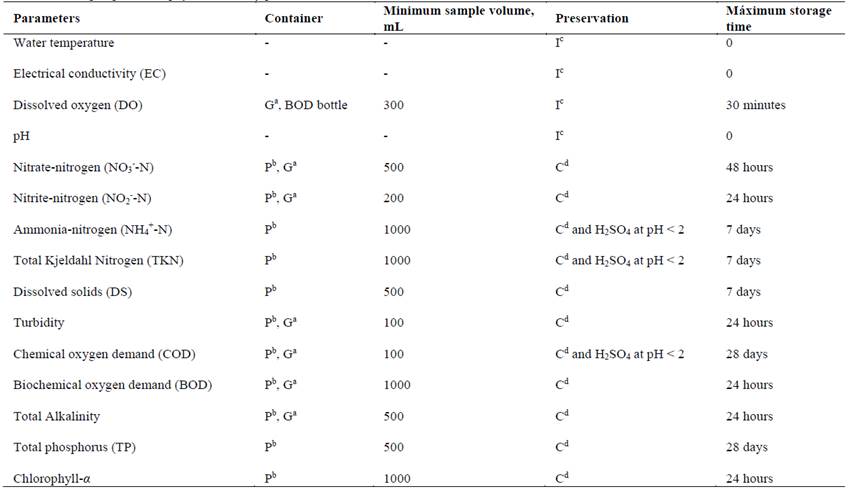
The conservation and handling of the samples were based on Mexican regulation NMX-AA-014-1980 [7]. The samples were preserved at low temperatures, between 4°±2°C. According to Table 2 it can be observed the main criteria taken in order to select the container, minimum collecting volume (by aliquot), preservation and maximum storage time of the water samples.
Table 2. Criteria for sampling to know physic-chemistry parameters of water.
a: G, glass; b: P, polyethylene; c: I, in situ; d: C, cooling
Source: As recommended by [7]
2.3. Water quality analysis
The physic-chemistry parameters were determined based on Mexican regulations: NMX-AA-007-SCFI-2000 (determination of temperatures); NMX-AA-093-SCFI-2000 (determination of Electric Conductivity); NMX-AA-012-SCF2001 (determining of dissolved oxygen); NMX-AA-008-SCFI-2000 (determination of pH); NMX-AA-079-SCFI-2001 (determination of nitrate); NMX-AA-099-SCFI-2006 (determination of nitrite); NMX-AA-026-SCFI-2001 (determination of total nitrogen); NMX-AA-036-SCFI-2001 (determination of total alkalinity); NMX-AA-034-SCFI-2001 (determination of dissolved solids); NMX-AA-030-SCFI-2001 (determination of chemistry request of oxygen); NMX-AA-028-SCFI-2001 (determination of biochemical request of oxygen); and NMX-AA-029-SCFI-2001 (determination of total phosphorus) [7-19].
2.4. Determination of soluble reactive phosphorus
In order to determine the solubility of reactive phosphorus in 20 ml sample of filtered water, 2ml of reactive 1 (see below) was added, and 0.4 ml of reactive 2 (see below), followed by a moderate shaking. Then, the sample was placed in darkness for 45 minutes; after this time color intensity was measured using a spectrophotometer UV/Vis to 880 nm [20].
Reactive 1: solution A. Dissolve 0.2 g of tartrate of p otassium and antimony in 500ml deionized water. Add 1 ml of sulfuric acid, then weight 11.2 g of ammonium molybdate of ammonium tetra-hydrated and dilute in solution A. Shake continuously to facilitate the complete dissolution of molybdate. Gauge the solution to 1L.
Reactive 2: dissolve 27 g of ascorbic acid in 500mL of deionized water. Solution mother of phosphate 1000 mg/L de PO4 - P: weight 4.390 g of di-acid phosphate of potassium and add deionized water up to 1L.
2.5. Determination of photosynthetic pigments
In order to determine Chlorophyll-a, a sample of 500mL of water was filtered through a glass filter Whatman GF/C of 25 mm diameter. The chlorophyll was extracted with 50 mL of acetone 90% during 48 hours in the darkness. From the measurement of absorbency checking with different wave longitudes, the concentration of chlorophyll-a was calculated by using the following formula:
2.6. Eutrophic state evaluation
The evaluation of TSI was determined by the concentration of PT (µg/L) and chlorophyll-a (µg/L). The calculation was made through the following equations:
This type of index will reduce the trophic state of any lagoon or lake (in a scale from 0 to 100) by trying to avoid the inherent subjectivity to the terms oligotrophic, mesotrophic and eutrophic. The Table 3 summarizes the scale of values in a trophic state of a water body [21].
2.7 The OCDE (Organización para la Cooperación y el Desarrollo Económico), Organization for Economic Cooperation and Development
In order to achieve the determination of the trophic state by means of trophic category sequences recommended by OCDE, the TP and chlorophyll-a concentration were measured (Table 4).
2.8 Trophic state TRIX
To determine the trophic state TRIX, the concentration of chlorophyll-a, percentage of dissolves oxygen saturation, the concentration of NO3 --N, NO2 --N, NH4 +-N and soluble reactive phosphorus have already been determined [22]. The estimations were made by the following mathematic expression:
Where each one of the four components represent a variable trophic state: Productivity factor
Chlor α = chlorophyll-α concentration - α (µg/L)
│%od = absolute value about percentage deviation of saturation dissolved oxygen, it means │100 od
And nutritional factor
NID = Dissolved inorganic nitrogen, calculated as:
The constant K= 1.5 y m=12/10=1.2 are values of scale and were introduced to adjust the lower limit value of the index and the spreading of the trophic scale already related, from 0 to 10 units TRIX (Table 5).
3. Results and discussion
3.1. Water Quality Analysis
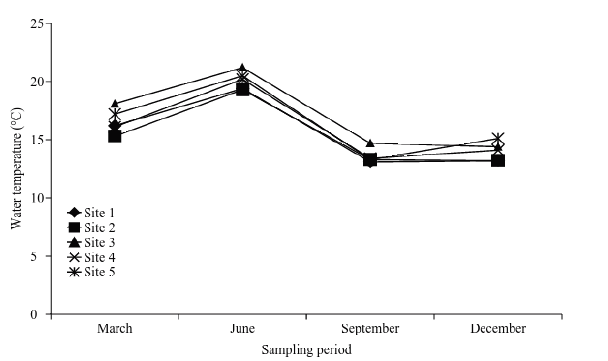
Table 6 summarizes the results obtained to determine the physic- chemistry parameters of the water of Tecocomulco´s lagoon. The average temperature of the water body indicates a spatial-temporary distribution with maximum values registered during June and minimums on winter (Fig. 2). The temperature varies between 13.1 to 21.2°C. The characteristic of the course (low deep, approximately 70 cm) suggests that most of the year the lagoon presents thermic homogeneity in depth.
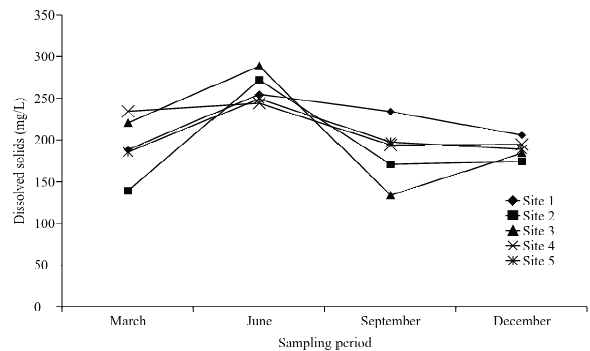
The average conductivity values in the months of March, June, September and December were of 253.111, 327.159, 196.755 and 211.148 µS/cm respectively. It is related to the concentration of dissolved solids (193.678, 261.853, 186.022 y 189.648 mg/L, in sites 1, 2, 3, 4 and 5, respectively), the temperature and the evaporation. The highest conductivity and dissolved solids values were obtained during the month of June, when the temperature was higher (Figs. 3, 4 and 5).
Source: The Authors.
Figure 4 Spatial-temporary distribution of water dissolved solid concentration.
Source: The Authors.
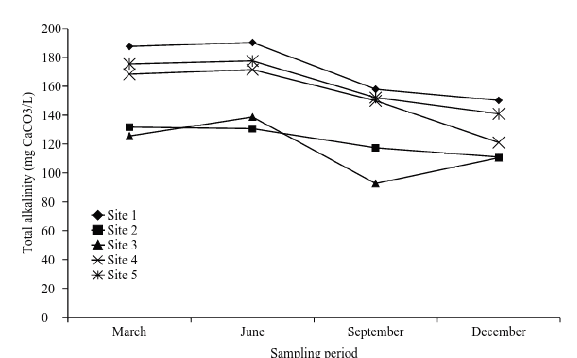
Figure 5 Spatial-temporary distribution of total alkalinity (low productivity < 75; medium productivity 75-150; high productivity > 150).
The determination of pH in the water body is a parameter to consider when it is necessary to analyze the chemistry speciation and solubility of organic and inorganic substances. Abiotic factors regulate half-full of the biologic processes by enzyme (photosynthesis, respiration); the availability of essential nutrients that limit the growth of the organisms (PO4 -3, NO3 -) and the mobility of heavy metals. Therefore, to understand the physic- chemistry behavior of the body water in lagoons this parameter seems very important.
Table 6 shows that during the first two periods of sampling, the water had a slightly acid pH values; this phenomenon was emphasized during the month of June because of the presence of CO2 produced by anaerobic bacterial respiration, the photosynthetic activity of submerged algae and aquatic macrophyte, and due to the decomposition of organic matter.
The variation of pH between periods and sampling sites could be explained by the diverse intrinsic and extrinsic factors such as:
1) Intrinsic factors:
a) Buffer capacity of the alkalinity carbonate and bicarbonate system.
b) Evaporation.
c) Intensity of biological processes such as photosynthesis, respiration and activities of organic matter decomposition.
2) Extrinsic factors:
a) Soil composition adjacent to the lagoon.
b) Pollution source: residual water spilled, influence of septic tank.
c) Temperature.
The pH is an important parameter; it is determined to identify the ionic species that contribute to water alkalinity. Alkalinity represents the main buffer system of water environments, and it plays an important role in the growth and development of algae and aquatic plants. It works as a reserve source to photosynthesis. Therefore, it could be used as an indicator of body water lagoons productivity, where high levels of alkalinity indicate high productivity.
The variability of values ca be attributed to diverse factors such as: the wearing away, rock and minerals dissolution, aerobic and anaerobic respiration processes and photosynthetic process, among others. Due to the values found in alkalinity (Table 6), it could be concluded that Tecocomilco´s lagoon has a high productivity (Fig.6), which can be confirmed through the ranking of eutrophication.
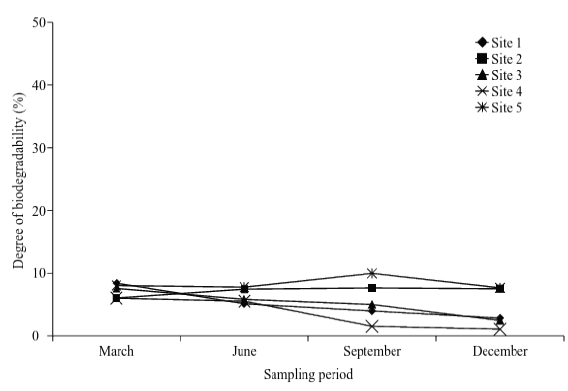
Source: The Authors.
Figure 6 Spatial-temporary distribution of Percentage and rank of organic matter biodegradability.
The parameter that shows wide-ranging in spatial and temporary differences is was DO. The level of DO is an indicator of the pollution rank and its capacity to support the vegetal and animal life. Commonly, a higher level of DO indicates better water quality; on the contrary, too low levels of DO mean that some biological species could not survive.
The sampling station with the lowest concentration of DO was site number 2, a place with high amount of Scirpus lacustris (common name tule), where hypoxia conditions are present (< 2.7 mg/L) during the first two periods of sampling, as a result of low water circulation, high temperatures, contribution of high organic matter content, and the consumption of oxygen during its composition.
The site number 5 had maximum values of DO (Table 6), showing submerged vegetation and high transparency, very important characteristic due to free oxygen during the process of submerged aquatic plants photosynthesis, a factor that contributes to increase the DO concentration.
A closely related parameter to DO is BOD, measured as the of amount of oxygen required to degrade organic matter in the water by microorganisms.
The process of waste decomposition will take place when the aquatic environments is polluted by organic matter like dead plants, residual water, organic waste from animals, and even food. When it happens, a lot of DO available is consumed by aerobic microorganisms, originating the reduction of big amounts of oxygen that can be used by other microorganisms. In this case, if DO is high, the level of BOD will be high too. Whereas organic waste is consumed the levels of BOD will get down. The water temperature can contribute to high levels of BOD. Once temperature increases, the speed of photosynthesis produced by algae and macrophytess usually increases as well in the water. When it happens the hydrophytes grow up and die quicker, making them to fall in depths where bacteria that usually require oxygen decompose them. So, the water with high temperature will accelerate the decomposition of bacteria and then will provoke high levels of BOD.
According to what was quoting before, it is possible to deduce that the sampling period with high levels of BOD5 is June. This information could be confirmed by the experimental determination of this parameter. During this month, the highest temperatures are present, as well as the lower levels of DO; it suggests that the microbial activity was high, and for this reason so was the BOD5. On the contrary, on December DO rises and the DBO5 was lower, due to lower temperatures.
The determination of COD and the calculus of biodegradability level (%) of organic waste (%BD=[BOD5/COD]*100) showed low values in all sites and sampling periods; these values are related and correspond to the rate BOD5/COD and it is an indication of a low percentage of biodegradability matter (Table 6). It means that a high proportion of dead material is refractory and tends to form humus compounds that attach to the sediments and it is going to represent another factor that can favor the obstruction of body water (Fig. 6 and Fig. 7).