INTRODUCTION
Alternative therapies using medicinal plants and herbal medicines have been growing worldwide, as natural treatments have low cost and less toxic effect compared to conventional drugs available. In addition, they also have a strong cultural influence. In Brazil, the popular use of medicinal plants is a common activity, involving inherited knowledge about the potential and specificity of plant species in combating diseases [1].
Herbal medicines have their action and effectiveness based on the way that the bio-active compounds present in the formulas and extracts can act. The pharmacological effect of natural products can be enhanced through synergistic interactions between the substances that compose them, optimizing the therapeutic action and reducing the necessary concentrations, then, consequently, minimizing the toxic effects related to these substances [2, 3].
Among the various Brazilian plant species used in natural therapies, the species of the Malvaceae family stand out, both from a chemical and pharmacological point of view. The species Waltheria viseosissima A. St. Hil., known as "malva viscosa", is used in folk medicine as an expectorant, antitussive and antihypertensive [4].
In this context, the execution of studies on Brazilian natural species applied as herbal medicines in the treatment of diseases contributes to the enrichment of the country's genetic and scientific heritage. Thus, the present study aimed to evaluate the in vitro cytotoxicity and ex-vivo genotoxicity of Brazilian species of the Malvaceae family: Waltheria viseosissima A. St. Hil.
METHODOLOGY
Plant material
The aerial parts of Waltheria viscosissima were collected in August 2013, in Santa Rita (Paraíba, Brazil-coordinates 07° 06'59" S and 34 ° 58'52" W). The plant was identified by Professor Maria de Fátima Agra from the Federal University of Paraíba (UFPB) and, later, registered and deposited at the Prof. Lauro Pires Xavier Herbarium (MF Agra number 21709) from UFPB (João Pessoa, Brazil).
The plant material was dried separately in an air oven at 40 °C, pulverized and macer ated with 95 % ethanol (5 L) for 72 hours. The extract solution was evaporated using the rotary evaporator under reduced pressure at 40 °C for the production of Crude Ethanol Extract (CEE). Then, the separation was performed by liquid-liquid chroma tography, using hexane, dichloromethane (CH2Cl2)/chloroform (CHCl3), ethyl ace tate and n-butanol, which resulted in the respective fractions, dichloromethane (DF) and chloroform (CF), in addition to the hydroalcoholic fraction.
The chloroform fraction (CF) was chromatographed in flash silica using petroleum ether, dichloromethane and methanol individually or in binary mixtures. The resulting polar fractions were chromatographed in Sephadex (LH-20), employing methanol and chloroform: methanol (1:1) as the mobile phase. All samples used were provided by the team of Professor Maria de Fátima Vanderlei de Souza (UFPB) and the procedures for obtaining and structural determination were described in previous works [5]. This research was registered in the Sistema Nacional de Gerenciamento de Recursos Genéti cos e Conhecimento Tradicional Associado" (SisGen-A568B8A).
Samples of oral mucosa cells and human erythrocytes
The tests were carried out in accordance with the World Medical Association Code of Ethics and approved by the Ethics Committee of the Centro Universitario de Patos (protocol number: 3,621,284). Blood samples (A, B and O) and smears of the oral mucosa were collected from healthy young adults, of both sexes, between 18 and 40 years of age.
Hemolytic activity
Human blood (types A, B and O) was mixed with 0.9 % NaCl (1:30) and centrifuged at 2500 rpm for 5 min. The process was repeated twice, and the pellet from the last centrifugation was resuspended in 0.9 % NaCl to produce a 0.5 % red blood cell (RBC) suspension, free of leukocytes and platelets. 2 mL of RBC suspensions were treated with CEE-Wv and CF-Wv, in concentrations of 50, 100, 500 and 1000 fzg/mL. The samples were incubated for 1 h at 22 ± 2 °C and kept in slow and continuous shaking (100 rpm). Subsequently, they were centrifuged at 2500 rpm for 5 min. Hemolysis was quantified by spectrophotometry at the maximum absorbance wavelength (540 nm) [6]. A RBC suspension was used as a negative control (0 % hemolysis) and another suspension, with 1 % Triton X-100, was used as a positive control (100 % hemolysis). Each test was performed in triplicate and the data were expressed in percentages that represent the arithmetic mean of three measures.
Antihemolytic activity
The evaluation of the osmotic fragility of the erythrocytes was performed with the suspension of erythrocytes 0.5 %. The assay used the concentrations of 50, 100, 500 and 1000 μg/mL of the extract CEE-Wv and Fraction CF-Wv, incubated in 2 mL of RBC suspension 0.5 %, for 1 h, at 22 ± 2 °C. The samples were centrifuged at 2500 rpm for 5 min and the supernatant discarded. The RBC was resuspended in a hypotonic 0.24 % NaCl solution for one hour at 22 ± 2 °C. After this period, the samples were centri fuged at 2500 rpm for 5 min and the hemolysis of the supernatant was quantified by spectrophotometry at 540 nm [7]. The RBC suspension was used as a negative control (0 % hemolysis) and another RBC suspension was added with the hypotonic NaCl 0.24 % solution as a positive control (100 % hemolysis).
Ex-vivo analysis
Genotoxic effects on oral mucosa cells
Epithelial cells were collected on both sides of the oral mucosa by means of a cytobrush (endocervical sample/cell collector), considered the most appropriate instrument for obtaining exfoliated cells from this region [8]. The cells were kept in a tube with 5 mL of 0.9 % NaCl (cell preservation medium) until the slides were prepared. Control cells were divided into two groups: treated with hydrogen peroxide (0.0005 %) (positive control) and those that did not receive treatment (negative control).
The cells were washed twice in saline, centrifuged for 10 min at 1500 rpm, and then kept in 5 mL of saline. The supernatant was removed. The samples were washed once more and exposed ex-vivo to the CEE-Wv extract and CF-Wv fraction, in different concentrations (50, 100, 500 and 1000 μg/mL), for 30 min. Then, they were centri fuged and the supernatant discarded. Before preparing the smears, the slides were pre heated to 37 °C and the cells homogenized in a vortex mixer. They were placed on the slides, dried at room temperature and fixed in methanol: acetic acid (3: 1) for 15 min [9]. The slides were kept at room temperature for 12 hours, after which they were immersed in distilled water for 1 min and stained in Giemsa 2 % for analysis under optical microscopy [10]. Cell toxicity was assessed by the presence of cell indicators such as micronucleus, binucleation, karyolysis, cariorrexis and macronucleus [11, 12]. Approximately 1000 cells were analyzed per slide.
Statistical analysis
The experiments were carried out in triplicate and the results expressed in percent ages that represent the arithmetic mean of three measures. Data were analyzed using one-way analysis of variance (Anova) and Bonferroni's post hoc test. The tests were performed using the GraphPadPrism software (version 6.0 for Windows, San Diego, CA-USA). The differences were considered significant when p ≤ 0.05.
RESULTS AND DISCUSSION
Red blood cells are cells highly vulnerable to toxic substances and free radicals. Changes in the environment cause instability and can lead to the rupture of membranes of this anucleated cell type, which also lacks organelles and mitochondria [13]. Analyzes of cytotoxic effects in vitro through hemolytic and antihemolytic potential show the per centage of hemolysis defined by Rangel et al. [6] as low (0 to 40 %), moderate (40 to 80 %), and high toxicity, when the percentage is above 80 %.
The hemolytic analysis of CEE-Wv showed a low level of hemolysis (< 40 %) in all eva luated concentrations (50 to 1000 μg/mL). The CF-Wv had a low to moderate index with hemolysis > 40 %, at a concentration of 1000 μg/mL. There was variation in cell lysis according to blood type in the evaluation of substances isolated from W. viseosissima, in which hemolysis occurred in the order A < O < B. FC-Wv at a concentration of 1000 μg/mL showed moderate hemolysis for blood type B and O, with lysis < 55 % and < 52 %, respectively. In relation to blood type A, a low rate of cytotoxicity was demonstrated, with lysis < 30 % (figure 1).
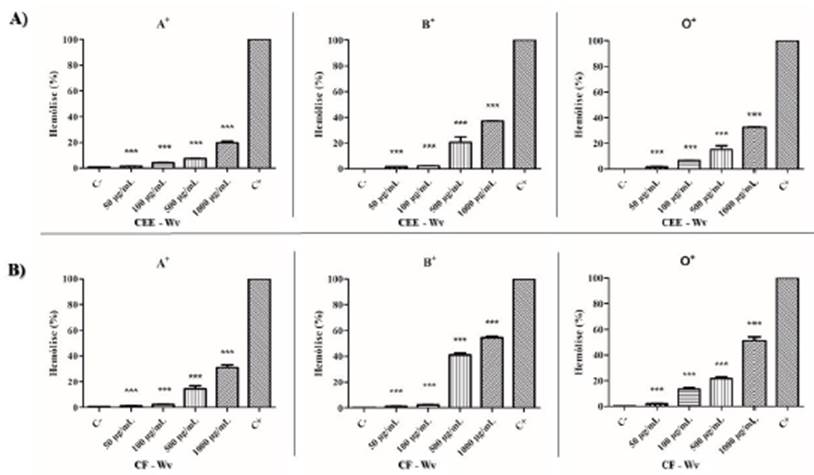
Figure 1 Hemolytic cytotoxic effect of CEE-Wv (A) and CF-Wv (B), products of W. viseosissima, against RBC; (C-) Negative control (RBC 0.5 %), (C +) Positive control (1 % Triton X-100). p <0.05 (*), p <0.01 (**) and p <0.001 (***) versus positive control.
In relation to other species of Waltheria, toxicity studies using extracts of leaves and roots of species W. indiea indicated the occurrence of low toxicity. In addition, phytochemical analysis indicated the presence of alkaloids, terpenes and flavonoids (tiliroside, quercetin, epicatechin), which have larvicidal activity [14]. Therefore, the cytotoxic content found in the CEE-Wv and CF-Wv products, isolated from W. viseosissima, suggests that these species have similarities in the supply of phytochemicals and in their pharmacological applications.
The protective effect of the products extracted from the species of the Malvaceae fam ily was verified through the anti-hemolytic potential in red blood cells of the ABO system. The extracts of W. viseosissima revealed that the CEE-Wv and CF-Wv showed good potential for stability and protection against hemolysis induced by NaCl 0.24 %, at concentrations of 500 and 1000 μg/mL. The lysis index was considered low to mod erate (26 % to 79 %). Blood type B had the lowest rate of hemolysis, and the percentage of lysis occurred in the order B < O < A (figure 2).
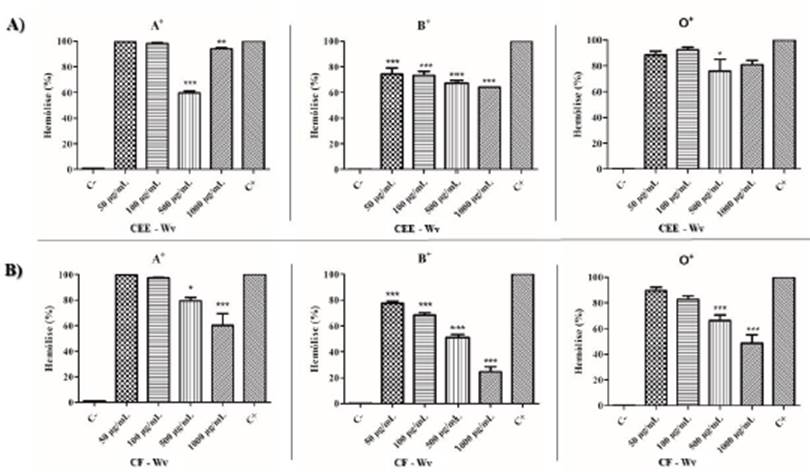
Figure 2 Cytotoxic anti-hemolytic effect of CEE-Wv (A) and CF-Wv (B), products of W. viseosis sima, against RBC; (C-) Negative control (RBC 0.5 %), (C +) Positive control (1 % Triton X-100). p <0.05 (*), p <0.01 (**) and p <0.001 (***) versus positive control.
The results obtained in the hemolytic and antihemolytic assay showed a slight diffe rence between the substances of the species belonging to the Malvaceae family in the blood types used (A, B and O). However, the variation in the intensity of cell lysis shows a trend of better interaction between the extracts evaluated and the antigenic portion of the red blood cells. The specific monosaccharides on the surface of the erythrocyte membrane are: type A (N-acetylgalactosamine), type B (D-galactose), type AB (has both antigens) and serotype O, which has no antigen on its surface [15].
The evaluation of cellular genotoxicity determines the effect of toxic agents when interacting with DNA, according to their probability of causing chromosomal damage, and structural changes that lead to cell death by necrosis/apoptosis [16]. Tests that analyze the risk of exposure to carcinogenic agents are used to determine the toxico logical risk of natural and synthetic substances. In the micronucleus test (comet assay), the exposure of squamous cells from the mucosa, are proposed as a method of assessing chromosomal damage in cells [17, 18].
Oral mucosa cells are important models for assessing DNA damage, as they are one of the first barriers against toxic and mutagenic substances. The analysis of genotoxicity using these cells confers many advantages, such as: primary exposure target, minimally invasive acquisition, monitoring of populations exposed to genotoxic agents, and allows associations between lifestyle [17].
The groups CEE-Wv and CF-Wv induced few cellular changes, when compared to the positive control, and presented a normal cell index >84 % at concentrations of 50 and 1000 μg/mL. The main changes presented by these groups were binucleation and macronuclei, cellular modifications compatible with induction of cell division activity. In addition, the CEE-Wv also showed rates of karyolysis compatible with the positive control at a concentration of 1000 μg/mL, suggesting induction of cell damage (table 1).
Table 1 Genotoxic profile of CEE-Wv and CF-Wv.
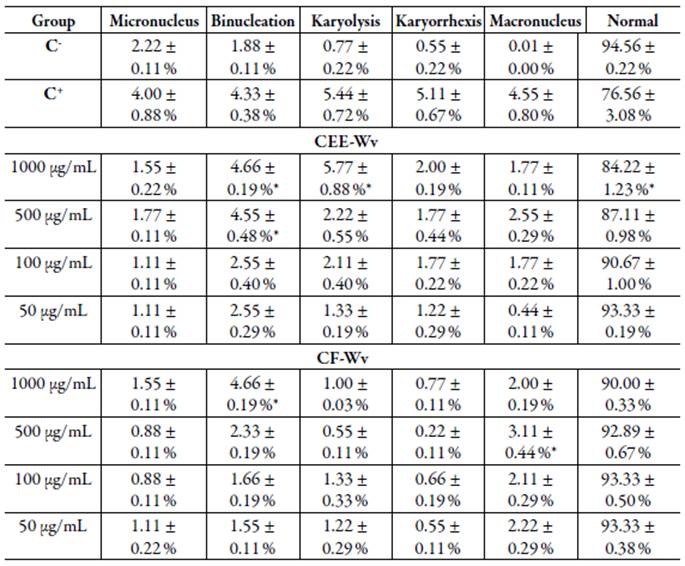
C-: Negative control; C+: Positive control; Values are mean ± standard deviation; * p <0.05 versus positive control.
The chromosomal changes that report the presence of macronuclei are more linked to cell activity, in which the process of division can be triggered by some agents, however there is no significant interaction or relationship with DNA. Binucleated cells are orig inated due to the difficulty in forming the mitotic spindle, caused by the aneugenic effect of some substances. Therefore, the presence of binucleation is significant to con firm cytological changes, but despite the interference of late events to cell division, no interactions with DNA occur [19, 20]. On the other hand, the occurrence of karyolysis is a nuclear alteration with significant toxicity. According to Tolbert et al. [19] and Hollanda et al. [17], it is indicative of necrosis and is related to cell death due to cyto toxic events, occurring frequently in inflammatory processes [21].
The products from the species belonging to the Malvaceae family - W. viseosissima -revealed to have low ex vivo genotoxic potential. The main cellular alterations found were binucleation, macronucleus and karyolysis. In phenolic compounds and flavonoids isolated from other species of the genera described in the present study, anti tumor and/or antineoplastic activities were observed in the extracts of W. ovata [22].
CONCLUSION
The evaluated parameters show that the products of the species W. viseosissima showed low cytotoxic and genotoxic potential. The products CEE-Wv and CF-Wv obtained the lowest cytotoxic potential with hemolysis below 40 % in all concentrations eval uated. The moderate anti-hemolytic effect was described in all samples evaluated at concentrations of 500 and 1000 μg/mL, with hemolysis < 60 %. In addition, the com pounds evaluated have low ex-vivo genotoxic potential, with a general index of normal cells greater than 84 %, even at higher concentrations (1000 μg/mL). The most fre quently observed changes were binucleation, macronucleus and karyolysis. The results showed that the natural products analyzed in this study have low toxicity, although a natural component is not free from harm to the organism, since it is used improperly. Thus, studies on toxicity are of great importance, as they can raise awareness about the abuse of medicinal plants.














