INTRODUCTION
Nanotechnology, also called the "science of the very small" allows creating nano-sized products and devices (10-9 m) with high functionality and specificity. Nanoformulated substances usually have chemical, physicochemical and biological properties different from those in their natural state [1]. In the last years, nanotechnological products have been developed for the treatment of cancer, inflammatory and cardiovascular diseases, diabetes, and obesity, among other conditions [2]. However, the selection of the poly meric material for nanoparticle preparation is a critical phase of this process.
Nanoparticle formulation arises as an effective way to improve solubility and bioavail ability of drugs belonging to groups II and IV of the biopharmaceutical classification system (BSC) [3]. The correct selection of the type and quantity of the coating poly mer determines important properties such as the site, the release profile, and the drug's physicochemical stability. Coating polymers must be non-toxic, hypoallergenic, and well tolerated by the intended route of administration (biocompatible) [1]. However, the variety of biocompatible film former polymers for nanoparticle preparation is still limited. Among the most used materials for this purpose are synthetic polymers such as those derived from methacrylic acid [4], poly-s-caprolactone, and polylactic acid [5, 6]. Polymers of natural origin such as chitosan, cellulose esters, alginates, and some others are also used [1]. However, synthetic polymers are the most appropriated for nanoparticle preparation. Polymers of natural origin usually produce unstable nanoparticles and make the preparation methodologies less reproducible [5].
Nanocapsules are drugs' carrier systems highly effective. Depending on the type of polymer and the characteristics of the coating layer that it forms, nanocapsules can be vectored towards a specific site (for example the intestine) and the drugs' release can be controlled [5]. Nanocapsules can transport drugs of both chemical-synthetic and natural origins such as plant extracts [7] and isolated and/or purified compounds. However, only some polymers have been shown a wide functionality that allows cov ering chemical-synthetic and natural-origin drugs, maintaining suitable stability and high functionality.
A versatile polymer with broad functionality that has been little used for nanoparticle preparation is Kollicoat MAE® 100P. This polymer has been used, successfully, to pre pare nanoparticles loaded with plant extracts [8], with drugs of chemical-synthetic ori gin [9], and nanocapsules loaded with a drug isolated from a plant oilresin. Thus, this review aims to update the state of knowledge about the physicochemical characteristics of the Kollicoat MAE® 100P, and its use as a film former polymeric material for prepar ing gastro-resistant nanoparticles. Physicochemical characteristics and the function ality of nanoparticles prepared with Kollicoat MAE® 100P were also reviewed. Thus, an exhaustive review (from 1980 to 2021) in various scientific databases like Medline, Scopus, EBSCO, and Cambridge, was performed.
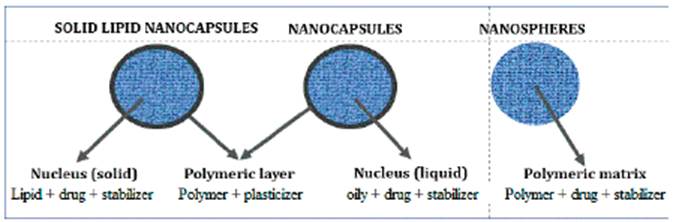
Nanocapsules
Nanocapsules are nano-sized vesicular particles (figure 1) with a polymeric layer surrounding a nucleus containing the drug [5]. When the nucleus is an oily liquid, they are called nanocapsules, but when the nucleus is a solid lipid, they are called solid lipid nanoparticles [10]. Nanocapsules with an aqueous liquid core are called polymersomes and are prepared using amphiphilic block copolymers [11]. The main characteristic of nanocapsules is the perfect differentiation between the coating shell and the core mate rial, contrary to nanospheres, where the polymeric material and the drug encapsulated form a matrix or lattice where it is impossible to differentiate the shell from the core [5].
The proper selection of the preparation method, the core components, and the coating polymer (and plasticizers) allows preparing the desired nanoparticle [5, 9]. The nucleus of the nanoparticle can contain vegetal extracts, drugs from the chemical-synthetic ori gin and/or isolated compounds. In all cases, the appropriate selection of the nucleus component and the covering material (polymer and plasticizers) makes it possible to vectorize the drug to a specific site of action and controlling the amount and rate of drug release. The preparation of polymeric nanoparticles from liquid plant extracts (fluid and soft) generally produces nanospheres.
A suitable polymer for nanoparticle preparation must protect the drug from external factors like temperature, light, and O2. This means that the polymeric layer needs to improve the nanoparticle's stability [7]. Physicochemical properties of polymers (e.g., acid-base behavior, solubility, and lipophilicity) determine nanocapsules stability and the drugs' release characteristics. A precise knowledge of the physic-chemical proper ties of the film former polymer allows knowing, in advance, the possible site of release and the possibility of controlling the drug release processes.
Copolymers of methacrylic acid-methacrylate esters are among the most used synthetic materials for the preparation of nanoparticles loaded with drugs of reduced solubility [12]. Within this group of polymers is the class of Kollicoat MAE* copolymers [13], which currently have their greatest application in the preparation of enteric-coated tablets. However, Kollicoat MAE®-grade polymers, despite their excellent properties for nanoparticle preparation, have been little used for this purpose.
Kollicoat classes
There are five classes of Kollicoat on the market: Kollicoat IR, Kollicoat Protect, Kol licoat MAE®, Kollicoat SR, and Kollicoat Smartseal [14]. The chemical composition and utility of these materials are shown in (table 1). Kollicoat IR class are copolymers of vinyl alcohol grafted with ethylene glycol [13]. Kollicoat Protect is a mixture of Kol licoat IR (~60%) and polyvinyl alcohol (40%) and has been claimed for moisture and oxygen protection [13]. Kollicoat SR 30D class was designed for sustained-release for mulations being an aqueous dispersion of polyvinyl acetate and polyvinyl pyrrolidone (K30) [15]. By their side, Kollicoat Smartseal 100 P, is a mixture of diethylamino ethyl methacrylate and methyl methacrylate (3:7). Kollicoat Smartseal 30D is a 30% aque ous dispersion of Kollicoat Smartseal 100P. This polymer is insoluble at neutral pH but soluble at pH<5 being suitable for taste masking and moisture protection [13]. Kollicoat MAE* class includes three polymers with similar composition: Kollicoat MAE® 100 55, Kollicoat MAE® 30DP (30% aqueous dispersion of Kollicoat MAE* 100 55), and Kollicoat MAE* 100P (pre-neutralized Kollicoat MAE® 100-55). All these poly mers are used in the Pharmaceutical Industry to prepare enteric-coated pellets for hard capsules and tablets [16]. Kollicoat MAE® 100P has shown great utility for preparing gastro-resistant nanoparticles, nonetheless, this functionality has not been claimed in the literature.
Table 1 Class of Kollicoat*, composition and their use in pharmaceuticals.
| Kollicoat Class | Type | Composition | Use | Reference |
|---|---|---|---|---|
| Kollicoat IR | IR | Polyethylene glycol-Polyvinyl alcohol ratio of 1:3 | -Coating tablets and capsules -Binder in tablets, granules, and pellets -Effervescent tablets -Sustained-release tablets | [15] |
| Pro tect | Kollicoat IR, 59.70 % Polyvinyl alcohol, 40% Silicon dioxide, 0,3 % | -Protective coating of tablets and capsules -Tastes masking | [15] | |
| Kollicoat MAE* | 30D | Methacrylic acid-ethyl acrylate co-polymer (1:1), 27.0 % Polysorbate 80, 2.3 % Sodium lauryl sulphate, 0.7 % Water, 70.0 % | -Preparation of enteric tablets, capsules, granules, and crystals -Mask unpleasant tastes -Protect from the effects of high humidity | [15] [17] |
| 100 55 | Methacrylic acid-ethyl acrylate copolymer (1:1), 97.0 % Polysorbate 80, 2.3 % Sodium lauryl sulphate 0.7 % | -Preparation of enteric tablets, capsules, granules, and crystals -Mask unpleasant tastes -Protect from the effects of high humidity -Nanocapsules film former -Nanoparticles controlled release | [15] [17] [18, 19] | |
| 100P | Methacrylic acid-ethyl acrylate copolymer (1:1), partially neutralized with 6 mol % NaOH, 97.0 % Polysorbate 80, 2.3 % Sodium lauryl sulphate, 0.7 % | |||
| Kollicoat SR | 30DP | Polyvinyl acetate, 27.0 % Povidone K30 (Kollidon 30), 2.7 % Sodium lauryl sulphate, 0.3 % Water, 70.0 % | -Sustained release matrix tablets, pellet, and granules, and gelatin hard capsules | [15] [20] |
| Kollicoat SmartS eal | 30DP | Methyl methacrylate and di(ethyl)aminoethyl methacrylate (7:3) copolymer. 30% aqueous polymer dispersion | -Aqueous film coating | [15] [20] |
| 100P | Methyl methacrylate and di(ethyl)aminoethyl methacrylate (7:3) copolymer (spray dried powder) | -Aqueous, and organic film coating | [15] |
Physicochemical characteristics
Kollicoat MAE® 100P (figure 2) is an anionic polymer, with a weak acid behavior having a molecular weight of about 250 000 AMU. This polymer dissolves in solutions of pH> 5.5 [21]. Kollicoat MAE® 100P contains polysorbate 80 as a plasticizer to diminish the friability of the film formed for tablet preparation [14]. However, additional plasticizers must be used to obtain a flexible and more stable coating layer for nanoparticle preparation. This material also contains sodium dodecyl sulfate as an emulsifier. Kollicoat MAE® 100P is partially neutralized (about 6 mol-% of the carboxyl groups) to facilitate its redispersion in water. This characteristic is especially useful for preparing nanodispersion of vegetal extracts. Kollicoat MAE® 100P complies with the current monograph of methacrylic acid-ethyl acrylate copolymer (1:1) [22] and is listed as "Partially neutralized methacrylic acid: ethyl acrylate copolymer" in the USP-NF [21].
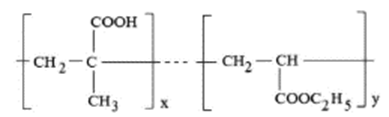
Figure 2 Basic structure of Kollicoat MAE* 100P copolymers formed by methacrylic acid: ethyl acrylate (x=y).
Kollicoat MAE® 100P forms aqueous dispersions due to is pre-neutralized with sodium hydroxide, without requiring a further addition of a basic solution [13, 16]. This polymer is soluble in ethanol and acetone (at 35-45 °C) in which forms a slightly viscous solution [16]. This property is used to prepare the organic phase for nanocap sules preparations. For nanocapsules, the organic phase (25-35%) is formed by Kolli coat MAE® 100P, the drug, the organic solvent, and a low HLB surfactant. When the organic phase is added to the aqueous phase (65-75%), which is composed of water plus a nonionic surfactant or another plasticizer, the Kollicoat MAE® 100P insolubilize enclosing the components of the organic phase for forming the nucleus. The change in polarity when the phases are mixes, followed by diffusion of the organic solvent (by stirring or by evaporation under vacuum) get hardened the polymeric shell around the nucleus containing the drug, to form the nanoparticle [23]. This procedure allows preparing nanocapsules loaded with water-insoluble drugs, such as loratadine [9] and a-amyrin [24].
Kollicoat MAE® 100P, when dispersed in water (at 20%) form stable particles with a mean diameter of approximately 300 nm [13]. However, the aqueous dispersion (2%) submitted at a high shear of 7000 rpm, for five minutes in an ultraturrax appa ratus (IKA T25, UK) produce nanoparticles of « 50 nm (figure 3) with a normal size distribution (Polydispersity index 0.198) [13, 16]. The excellent dispersibility of this partially neutralized polymer in aqueous solutions is an excellent characteristic for nanoparticle preparation that is still little explored.
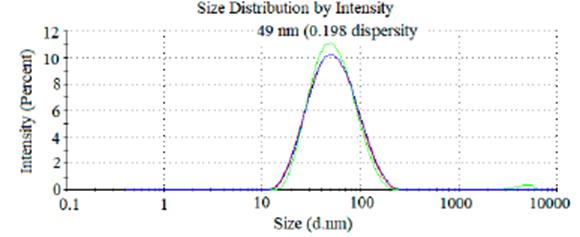
Figure 3 Particle size of 2 % Kollicoat MAE* 100P aqueous nanodispersion (authors' unpublished work).
Kollicoat MAE® 100P forms a brittle film that breaks at an elongation under one per cent [13]. For this reason, plasticizers are essential for producing a coating layer with suitable elasticity to achieve good stability. Hydrophilic plasticizers like propylene gly col, triethyl citrate (TEC), as well as non-ionic surfactants (e.g., Brijs, polysorbates, and ethoxylated fatty acid esters), favor the elasticity of the coating layer and improve the stability of the nanocapsules shell. Hydrophilic plasticizers give flexibility and resist ance to the coating layer and enhance the dispersion in the aqueous medium, favoring the interaction with the polymeric chain of the Kollicoat MAE® 100P [13].
Nanoformulations with Kollicoat MAE® 100P
The nanodispersion of Cassia grandis L. f [19] was developed using the polymer dep osition-solvent diffusion method [18]. This methodology allowed obtaining a mean particle size of 75 nm, with a closed polydispersity index of 0.176, and a zeta potential of -11.20 mV. With the same methodology, it was prepared nanoparticles of loratadine, a chemical-synthetic drug [19], which exhibited a mean particle size of 43 nm, with a polydispersity index of 0.250 and -19.30 mV of zeta potential. In the same way, it was prepared nanocapsules loaded with a-amyrin (a hydrophobic drug obtained by isolation and purification from a vegetal oilresin [24]. Nanocapsules loaded with a-amyrin showed particle size of 130 nm, polydispersity index 0.100, and -38.65 mV of zeta potential. The abovementioned nanoformulations showed small particle sizes (between 43 and 130) with normal size distribution and high modular values of zeta potential. These results suggest that Kollicoat MAE® 100P is a versatile polymer, which allows preparing nanoformulation loaded with drugs from diverse origins, resulting nanosystems with homogeneous sizes, which is essential for achieving good stability.
Properties of Kollicoat MAE® 100P loaded nanoparticles
The development of nanoparticles loaded with plant extracts is considered a technolog ical challenge. Plant extracts are complex mixtures of metabolites that present different characteristic of solubility, permeability, and stability [1]. The development of nano particles containing plant extracts improves the pharmacological effect of the extracts enhancing the stability and allows optimize their use since lower doses are needed to achieve the desired effect. The use of biocompatible polymers to produce a coating layer to protect metabolites present in the extracts by the formation of nanocapsules or a polymeric matrix (nanospheres), is an innovative and highly effective technology to improve bioavailability and stability of medicinal plant extracts, which, however, is still sub explored.
Prada and co-workers [8, 25] prepared nanoparticles of Cassia grandis (a medicinal plant traditionally used for the treatment of Diabetes mellitus, an anemias) coated with Kollicoat MAE® 100P. In that study, three nanoparticle formulations were devel oped using three different film former polymers (Eudragit® L, PEG 4000, and Kolli coat MAE® 100P). The nanoformulations particle size, polydispersity index, and zeta potential were compared, as well as the stability. Nanoparticles coated with Kollicoat MAE® 100P showed the smaller particle size, a lower polydispersity index, and the higher modular value of Zeta potential. The encapsulation efficiency of the process was greater than 85 % [25] and the loading capacity was 73 %. Kollicoat MAE® 100P coated nanoparticles showed the best performances showing the ability of this poly mer to produce nanoparticles loaded with vegetal extracts.
Several studies demonstrated the stability, in the stomach environment, of nanoparticles coated with Kollicoat MAE® 100P [9, 24, 26]. Drugs with erratic absorption in the stomach, or that undergo transformations that reduce their bioavailability need to be formulated, so that their release and absorption occur in the intestine. Loratadine is a drug that belongs to group II of the BSC classification system [26]. This drug is protonated in the acidic environment of the stomach, decreasing its absorption [9]. For this reason, a nanoparticle formulation for intestinal release of loratadine was prepared using Kollicoat MAE® 100P as a coating polymer [9]. Very stable nanoparticles, «75 nm in size and with a normal distribution were obtained. An encapsulation efficiency greater than 84% was obtained. This study confirmed the suitability of Kollicoat MAE® 100P as a gastro-resistant shell-forming polymer in the preparation of nanoparticles, a characteristic that had not been reported in the literature.
Effect of temperature on nanoparticles coated with Kollicoat MAE® 100P
In 2006, a study reported the deformation capacity of polymeric films prepared with Kollicoat MAE® 30DP, in the presence and absence of Polysorbate 80, used as a plas ticizer [4]. Polysorbate 80 improved the film elasticity, allowing it to withstand defor mation forces 5 times greater than those films that did not contain this plasticizer. As a result, the combination of Kollicoat MAE® 30D-Polysorbate 80 produced flexible films with high thermal stability. A study conducted by our working group evaluated the effect of the temperature (from 20 to 70 °C) on the size and size distribution (poly-dispersity index) of Kollicoat MAE® 100P nanoparticles loaded with a plant extract [25] and with a synthetic drug [18]. In both cases, Polysorbate 80 was used as the plasticizer. Temperatures between 20 and 60 °C did not modify, significantly, the parti cle size of both nanoformulations. Temperatures greater than 60 °C produced a 300% increase in size on the nanoparticles loaded with the plant extract (from 200 to 650 nm), while in nanoparticles loaded with the synthetic drug the size remained practi cally constant (32-35 nm). However, between 20-60 °C, the polydispersity index of both formulations remained low (0.020-0.140 in the plant extract-loaded nanodisper sion and between 0.260 and 0.280 in the nanoparticles of the synthetic drug). These studies showed that, despite the particle size deformation produced by the temperature, there was slight variation in the particle size distribution. This fact indicates the high thermal stability of nanoparticles coated with Kollicoat MAE® 100P (between 20 and 60 °C), regarding the nature of the enclosed drug.
It is important to highlight that nanoparticle preparation methods can be of low or high energy input [19]. Some preparation processes and some unitary operations (e.g., ultrasonic stirring) significantly increase the temperature of the liquid medium con taining nanoparticles preparation. Under these conditions, it is convenient to use a biocompatible polymer, such as Kollicoat MAE® 100P, which produces thermoresist ant coated films and improves the stability of nanoformulations, regardless of the ori gin of the nanoformulated drug. However, the strength, elasticity, and stability of the coating layers formed by Kollicoat MAE® 100P depend on the plasticizer.
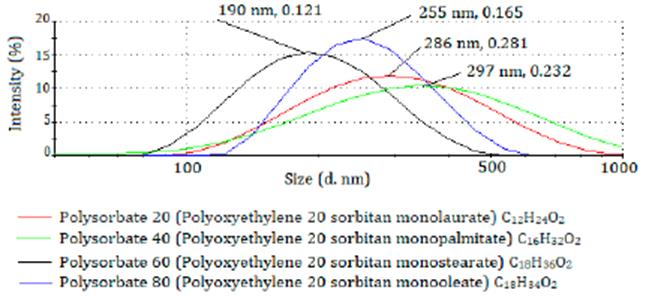
In an unpublished work of our working group it was evaluated the effect of the carbon chain length of the fatty acid present in the sorbitan ester (non-ionic surfactant) on the particle size and size distribution of nanoparticles coated with Kollicoat MAE® 100P. Four white nanodispersions (without active ingredient) were prepared by the polymer deposition-solvent diffusion method [22] using 750 mg of Kollicoat MAE® 100P in 5 mL of ethanol: acetone mixture 1:1 (organic phase) and 500 mg of each polysorbate in 100 mL of distilled water. In all cases, it was used the same experimental procedure. As the length of the carbon chain of the esterified acid increases, the particle size and poly dispersity index decreased (figure 4). This experiment suggested the feasibility of using other sorbitan esters as plasticizers jointly with the Kollicoat MAE® 100P in nanopar ticle preparation. Thus, it would be necessary to evaluate the effect of combinations of Kollicoat MAE® 100P with plasticizers of different nature (example: polyethylene glycol, and TEC) and/or the effect of other polymers like polyvinyl alcohol on the physic-chemical characteristics, release profiles, and stability of nanoparticles loaded with active ingredients of diverse nature.
Release profile of Kollicoat MAE® 100P-loaded nanoparticles
Nanoparticle formulations produced with Kollicoat MAE® 100P as a coating poly mer did not show prolonged release profiles at pH between 6.5-7.4 [9, 26]. The Cassia grandis nanoparticles, in simulated intestinal fluid (pH 6.5) with and without pancreatin (pH 6.5 and 7.4, respectively) released 100% of the active ingredient in 70 min [8], while loratadine nanoparticles (in the same fluids) released 100% of the drug in 40 min. These studies confirmed the ability of the nanoparticles produced with Kollicoat MAE® 100P to maintain, for at least 40 min, the drug release at pHs compatibles with the intestinal fluid in fasted and fed condition.
These results suggest the need to conduct other studies combining Kollicoat MAE® 100P with different plasticizers and stabilizers, for controlling, with precision and reproducibility the release of the drug nanoformulated. Nonetheless, broad ranges of drug release were observed with the use of methacrylic acid copolymers combined with some organic compounds, such as ethylcellulose [27].
On the other side, Kollicoat MAE® 100P was used to form a gastro-resistant film in hard gelatin capsules filled with rHuKGF protein. The study determined the absolute bioavailability of this protein in gastro-resistant capsules, comparing it with intrave nous administration. After the oral administration, the rHuKGF protein formulated in gastro-resistant capsules showed an absolute bioavailability of 74%. The study demonstrated that the protein was adequately released after the dissolution of Kolli coat MAE® 100P, successfully crossing the intestinal membranes to reach the systemic circulation [28].
Pharmacological properties of nanoparticles coated with Kollicoat MAE® 100P
One of the most advantages of nanoparticles is that they can modify the activity of drugs. Cassia grandis nanoparticles coated with Kollicoat MAE® 100P showed (in vitro) an antioxidant effect more potent than the extract and the gallic acid (used as a control) [8]. On the other side, nanoparticles loaded with Cassia grandis extract pro duced a strong inhibition of the pancreatic lipase, however, the extract did not inhibit pancreatic lipase. Similarly, Cassia grandis nanodispersion showed (in vivo) a hypo glycemic potency 20-fold higher than the crude extract. The authors suggest that the particle size in nanometric scale and the slow release of the drug from nanoparticles potentiate the pharmacological effect. These results confirm the need for research to improve the release properties of nanoparticle coated with Kollicoat MAE® 100P, in order to obtain a better pharmacological performance, optimizing and rationalizing the use of drugs of any nature that could be nanoformulated.
A gastro-resistant nanocapsules formulation of glibenclamide coated with methacrylic acid-ethyl acrylate copolymer showed a hypoglycemic effect (in vivo) five times greater than the commercial form of this drug [29]. This, is one of the most significant char acteristics of nanoparticle-based on this kind of polymers, potentiating the pharmaco logical effect of the drugs.
Another highlighted characteristic of the Kollicoat MAE® 100P is the innocuousness. Synthetic polymers and copolymers based on methacrylic acid tested as drug carriers of different drugs do not exert either toxic effects or changes in the metabolic activity of immune system cells [30]. The innocuity was also observed in immunogenicity studies performed on coated tablets with methacrylic acid. The study showed that the films formed by this polymer do not produce any abnormal response of the immune system [31].
CONCLUSION
Investigations have shown that Kollicoat MAE® 100P is a versatile polymer that can be used for the preparation of gastroresistant nanoparticles with drugs of diverse origins. With this polymer can be prepared nanoparticles with size smaller than 130 nm and low polydispersity indexes, and relatively high z-potentials, which confers a great stability to nanoparticle system. Nanoparticles produced with Kollicoat MAE® 100P combined with a suitable plasticizer exhibit a hard and flexible shell and contribute to sustained drug release systems. Besides, drugs nanoformulated with Kollicoat MAE® 100P as coat ing polymer are insoluble at pH<5.5 and presented a good thermal stability at temper atures of up to 60 °C. This review confirmed that Kollicoat MAE® 100P, despite being a well-known polymer in the Pharmaceutical Industry, has still been little investigated for nanoparticles preparations. Kollicoat MAE® 100P represents a viable, low-cost, and multifunctional alternative for the preparation of nanoparticle systems, which requires more studies in its use, to build nanoformulated products with better performances.