Introduction
COVID-19 is a disease that spreads quickly and has a high transmission efficiency. It was declared a pandemic by the World Health Organization in March 2020 due to the rapid increase in cases worldwide and the high rate of hospitalization and admissions to intensive care units it caused after being identified. As of September 28, 2021, it had already caused some 232 million infections and more than 3 million deaths worldwide.1,2 In Colombia, as of May 31, 2022, 6 103 455 cases and 139 854 deaths had been reported;3 however, studies are scarce and there is not much data on the variables related to greater severity of the disease in the region.4,5
In a systematic review including 77 studies from Europe, China, and the United States, Dorjee et al.6 found an overall prevalence of death from COVID-19 of 20%, with relative risk of death of 3.6 (95%CI: 3.0-4.4) for age ≥60 years, 1.3 (95%CI: 1.2-1.4) for male sex, 1.3 (95%CI: 1.1-1.6) for smoking history, 1.8 (95%CI: 1.6-2.0) for hypertension, 1.7 (95%CI: 1.4-2.0) for chronic obstructive pulmonary disease, 1.5 (95%CI: 1.4-1.7) for diabetes, 2.1 (95%CI: 1.8-2.4) for heart disease, and 2.5 (95%CI: 2.1-3.0) for chronic kidney disease. It has also been reported that there are laboratory and imaging findings associated with adverse outcomes.7-12In this regard, it has been established that patients with COVID-19 who require admission to the ICU have elevated levels of neutrophils, aspartate aminotransferase (AST) and C-reactive protein (CRP) on admission, and that lymphopenia as well as elevated lactate dehydrogenase (LDH) and creatinine kinase have been identified as possible markers of severity.10
Concerning imaging findings, it has been reported that ground-glass opacities are a consequence of diffuse alveolar damage, which is associated with the pathogenesis of viral infections,7 and that there is a correlation between the extent of radiological involvement and the severity of the disease.13
Studying clinical characteristics and outcomes during the early stages of outbreaks of any disease contributes to the knowledge of the disease and provides tools to optimize prevention, diagnosis, and treatment strategies.14 In this sense, the objective of the present study was to compare the sociodemographic, clinical, imaging, and laboratory characteristics of patients diagnosed with COVID-19 treated at the emergency department of a clinic in Cali, Colombia, based on the requirement for admission to the intensive care unit (ICU).
Materials and methods
Study type and population
Retrospective, descriptive, single-cohort study. The study population comprised all adult patients (>18 years) with COVID-19 (diagnosis confirmed by reverse transcriptase polymerase chain reaction test) with symptoms and admitted between March and April 2020 to the emergency department of a quaternary care hospital in Cali (N=66). Once the medical records were reviewed, 17 patients who received outpatient treatment were excluded because their follow-up data were incomplete, so the final sample was made up of 49 participants.
Study procedures and variables
Patients were classified into two groups depending on the place where they received treatment: patients requiring ICU admission (ICU requirement: n=24) and patients treated in general wards or on an outpatient basis (No ICU requirement: n=25).
The variables analyzed in the study were sex; age; type of health insurance coverage; comorbidities; pharmacological history; symptoms, time of evolution, laboratory and imaging findings on admission to the emergency department; medications used in the institution for the treatment of SARS-CoV-2 infection in the initial stage of the pandemic (lopinavir/ritonavir 500/125mg every 12 hours, hydroxychloroquine 200mg every 12 hours, or chloroquine 500mg every 12 hours, ivermectin at a dose of 1-2 drops per kilo/day for 5 days); antimicrobials used for the treatment of bacterial superinfection; and imaging findings (chest X-rays and CT scans). All data were taken from the patients' medical records.
The outcomes considered in the study were oxygen requirement by nasal cannula and high-flow nasal cannula, invasive mechanical ventilation (IMV), development of complications, and death.
Statistical analysis
The data for each variable were entered in an electronic form in Google Forms by health personnel trained for this purpose. Qualitative variables were summarized using absolute frequencies and percentages, and quantitative variables were summarized using measures of central tendency (means and medians) and dispersion [standard deviation (SD), interquartile range (IQR)], according to their distribution (Shapiro-Wilk test). For qualitative variables with more than one response option, each category was analyzed independently.
Bivariate analyses were performed to determine the differences between both groups using the chi-square or Fisher's exact test for quantitative variables, and Student's t test for qualitative variables. Nonparametric Mann-Whitney U test was used in cases in which no normal distribution was observed. All analyses were performed using R statistical software version 4.0.3,11 and a significance level of p<0.05 was considered.
Ethical considerations
The study was approved by the Institutional Research Ethics Committee of Clínica Imbanaco in accordance with Minutes No. 243 of April 30, 2020. Moreover, the ethical principles for research involving human subjects established in the Declaration of Helsinki15 and the provisions on health research contained in Resolution 8430 of 1993 of the Colombian Ministry of Health were taken into account.16
Results
The majority (59.18%) of participants were male and the mean age was 53 years (range: 23-86 years, SD=14.98). Table 1 describes the demographic and clinical characteristics of the included patients.
Table 1 Sociodemographic and clinical characteristics of the sample on admission to the emergency department.
| Characteristics | Total n=49 | ICU n=24 | Non-ICU n=25 | p-value | |
|---|---|---|---|---|---|
| Sex (n, %) | Female | 20 (40.81) | 9 (37.50) | 11 (44.00) | 0.773 |
| Male | 29 (59.18) | 15 (62.50) | 14 (56.00) | ||
| Age in years (mean, SD) | 53 (14.98) | 53 (14.98) | 58 (13) | 48 (15) | |
| Age by category (n, %) | <60 years | 34 (69.39) | 14 (58.33) | 20 (80.00) | 0.128 |
| ≥60 years | 15 (30.61) | 10 (41.67) | 5 (20.00) | ||
| Type of health insurance coverage (n, %) | Contributory | 16 (32.65) | 9 (37.50) | 7 (28.00) | 0.551 |
| Prepaid medicine | 19 (38.77) | 6 (25.00) | 13 (52.00) | 0.079 | |
| Private | 4 (8.16) | 1 (4.16) | 3 (12.00) | 0.609 | |
| Occupational hazards | 1 (2.04) | 0 (0) | 1 (4.00) | - | |
| Subsidized | 9 (18.36) | 8 (33.33) | 1 (4.00) | 0.010 | |
| Comorbidities (n, %) | Arterial hypertension | 19 (38.77) | 13 (54.16) | 6 (24.00) | 0.042 |
| Obesity | 16 (32.65) | 9 (37.50) | 7 (28.00) | 0.5512 | |
| Diabetes | 9 (18.36) | 8 (33.33) | 1 (4.00) | 0.010 | |
| Asthma | 1 (2.04) | 0 (0) | 1 (4.00) | - | |
| Kidney failure | 1 (2.04) | 1 (4.16) | 0 (0) | - | |
| Smoking | 3 (6.12) | 2 (8.33) | 1 (4.00) | 0.609 | |
| Cardiovascular disease | 3 (6.12) | 3 (12.50) | 0 (0) | - | |
| Cancer | 4 (8.16) | 4 (16.66) | 0 (0) | - | |
| Rheumatologic disease | 2 (4.08) | 1 (4.16) | 1 (4.00) | - | |
| Pharmacological history (n, %) | Chemotherapeutic agents in the last three months | 1 (2.04) | 1 (4.16) | 0 (0) | - |
| Systemic steroids | 4 (8.16) | 3 (12.50) | 1 (4.00) | 0.3487 | |
| Angiotensin converters inhibitors | 6 (12.24) | 4 (16.66) | 2 (8.00) | 0.417 | |
| Angiotensin II receptor blockers | 7 (14.28) | 4 (16.66) | 3 (12.00) | 0.701 | |
| Other antihypertensives | 3 (6.12) | 1 (4.16) | 2 (8.00) | 1 | |
| Other pharmacological history | 14 (28.57) | 9 (37.50) | 5 (20.00) | 0.216 | |
| Signs and symptoms on admission (n, %) | Cough | 40 (81.63) | 18 (75.00) | 22 (88.00) | 0.289 |
| Fever | 35 (71.42) | 16 (66.66) | 19 (76.00) | 0.538 | |
| Odynophagia | 16 (32.65) | 5 (20.83) | 11 (44.00) | 0.128 | |
| Respiratory distress | 31 (63.26) | 20 (83.33) | 11 (44.00) | 0.007 | |
| Fatigue or adynamia | 38 (77.55) | 17 (70.83) | 21 (84.00) | 0.320 | |
| Others | 32 (65.30) | 13 (54.16) | 19 (76.00) | 0.139 | |
| Body temperature in °C (Median, P25-P75) | 37.0 (36.538.0) | 37.0 (36.538.0) | - | - | |
| Symptom evolution in days (Median, P25-P75) | 8 (5-10) | 8 (5-10) | - | - | |
ICU: intensive care unit.
Source: Own elaboration.
Regarding clinical characteristics, the most frequent symptoms were: cough (81.63%), adynamia (77.55%), fever (71.42%), and respiratory distress (63.26%). The mean arterial oxygen pressure (PaO2) was 71.58 mmHg (SD=15.73) for patients who required ICU admission and 76.91 mmHg (SD=17.41) for those who did not. Only 14 (28.57%) patients had a fever >38°C on admission to the emergency department. 44 (89.79%) patients reported comorbidities at the time of consultation, the most common being arterial hypertension (n=19, 38.77%), obesity (n=16, 32.65%), and diabetes (n=9, 18.36%). Of the patients admitted to the ICU (n=24), the majority (62.5%) were males, and the mean age was 58 years (range: 25-83, SD=13).
Imaging findings
All participants underwent chest x-rays and 28 underwent chest CT scan at the emergency department. In both studies, ground-glass patterns and unilateral or bilateral areas of consolidation were reported; the frequency of these findings was higher in patients requiring ICU admission (Table 2). It should be noted that the latter finding presented statistically significant differences between both groups in the x-rays (p=0.005), but not in the CT scans.
Table 2 Imaging and laboratory findings of participants on admission to the emergency department.
| Test | ICU (n=24) n (%) | Non-ICU (n=25) n (%) | p- value | |
|---|---|---|---|---|
| Chest x-ray | Normal | 0 | 8 (32) | - |
| Consolidation areas (unilateral or bilateral) | 12 (50) | 3 (12) | 0.005 | |
| Peripheral ground-glass opacity, bilateral round opacity, or other pattern | 5 (20.8) | 2 (8) | 0.246 | |
| Pleural effusion | 1 (4.2) | 0 | - | |
| Nonspecific findings | 20 (83.3) | 14 (56) | 0.110 | |
| Chest CT scan | Normal | 0 | 2 (8) | - |
| Unilateral or bilateral consolidation | 7 (29.2) | 5 (20) | 0.520 | |
| Peripheral ground-glass opacity, bilateral round opacity, or other pattern | 12 (50) | 11 (44) | 0.777 | |
| Crazy-paving pattern | 0 | 1 (4) | - | |
| Pleural effusion | 1 (4.2) | 0 | - | |
| Nonspecific findings | 4 (16.7) | 3 (12) | 1 | |
| Hemogram | Leukocytes (mm3) * | 7 570 (6 497.5-10 397.5) | 5 130 (4 540-6 390) | 0.0013 |
| Lymphocytes (mm3) * | 865 (600-1 102.5) | 1 400 (1 050-1 990) | <0.0001 | |
| Hemoglobin (mg/dL) t | 13.70 (1.51) | 13.96 (1.73) | 0.5541 | |
| Neutrophils (mm3) * | 5980 (4650-8147.5) | 3450 (2720-4140) | <0.0001 | |
| Platelets (platelets/uL) * | 247 000 (168 750-283 000) | 210 000 (171000-258 000) | 0.35 | |
| Other paraclinical tests | C-reactive protein (mg/L) * | 141.25 (71.42-203.57) | 27.95 (8.98-48.88) | <0.0001 |
| Creatinine (mg/dL) * | 0.82 (0.665-1.275) | 0.85 (0.70-0.98) | 0.724 | |
| Procalcitonin (mg/mL) * | 0.3950 (0.142-1.117) | ND | ||
| Aminotransferase aspartate-ASAT (U/L) * | 34 (27-55) | 26 (23-61) | 0.4221 | |
| Alanine aminotransferase-ALAT (U/L) * | 26.5 (20-50.5) | 32 (14.5-54.5) | 0.8526 | |
| Total creatine kinase (mg/dL) t | 64.82 (54.97) | ND | ||
| Lactic acid | 1.615 (1.25-2.20) | 1.55 (1.26-2) | 0.9433 | |
| Ferritin * | 1 038 (679-2 000) | 542.5 (340.25-1 063.75) | 0.0073 | |
| Lactate dehydrogenase * | 391 (259.5-492.5) | 248.5 (198.75-294.25) | 0.0014 | |
ICU: intensive care unit; ND: no data. * Median (P25-P75). t Mean (standard deviation).
Source: Own elaboration.
Laboratory findings
Table 2 describes the laboratory results per study group. It reports that the median leukocyte, neutrophil, CRP, ferritin and LDH counts were higher in patients admitted to the ICU, while the median lymphocyte count was lower in this group.
Furthermore, variability differences were observed in the parameters evaluated between both groups: the distribution of leukocyte count (ICU: IQR=3 900/mm3 , Non-ICU: IQR=1 850/mm3) and neutrophil count (ICU: IQR=3 497.5/mm3, Non-ICU: IQR=1 420/mm3) was more variable in patients admitted to the ICU, while the distribution of lymphocyte count was more variable in those who did not require intensive care (ICU: IQR=502.5/mm3, non-ICU: IQR=940/mm3). Likewise, in patients admitted to the ICU, more variability in CRP (ICU: IQR=132.15 mg/L, non-ICU: IQR=39.9 mg/L), LDH (ICU: IQR=233 U/L, non-ICU: IQR=95.5U/L) and ferritin (ICU: IQR=1 321.5 ng/L, non-ICU: IQR=723.5 ng/L) levels was found. Figures 1 and 2 graphically represent these differences for each variable.
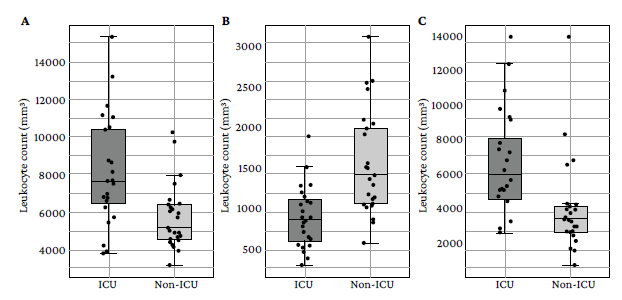
Source: Own elaboration.
Figure 1 Distribution of leukocyte (A), lymphocyte (B) and neutrophil (C) levels in the initial blood count of the study participants.
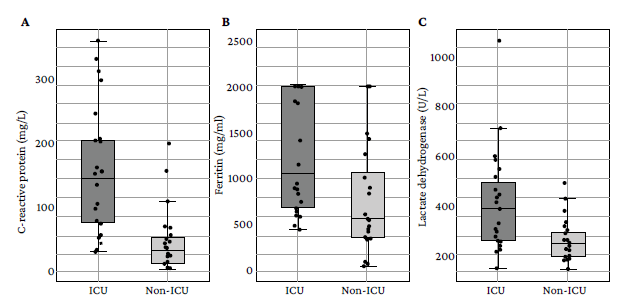
Source: Own elaboration.
Figure 2 Distribution of C-reactive protein (A), ferritin (B) and lactate dehydrogenase (C) levels in the study participants.
The results related to treatment and complications are shown in Table 3. It reports that the most frequent complication was bacterial superinfection, followed by kidney failure; that 5 patients died and 4 of them presented multiple organ system failure; and that of the 15 patients who required IMV, extubation failure was observed in 2 and one required tracheostomy. The most frequently prescribed drugs were hydroxychloroquine and chloroquine, and the combination lopinavir/ritonavir. Finally, 8 patients were treated on an outpatient basis and were not prescribed any specific therapy against COVID-19.
Table 3 Treatments and outcomes implemented in the study participants.
| Treatment/outcome | ICU (n=24) n (%) | Non-ICU (n=25) n (%) | |
|---|---|---|---|
| Complications | None | 8 (33.3) | 23 (92) |
| Kidney failure | 8 (33.3) | 0 | |
| Shock | 5 (20.83) | 0 | |
| Disseminated intravascular coagulation | 1 (4.17) | 0 | |
| Pulmonary thromboembolism | 2 (8.33) | 0 | |
| Bacterial superinfection (bacterial pneumonia documented by means of bronchoalveolar lavage, urinary tract infection, or bacteremia) | 9 (37.5) | 0 | |
| Multiple organ system failure | 5 (20.83) | 0 | |
| Other complications | 3 (12.5) | 2 | |
| Medications | Lopinavir/ritonavir | 0 | 10 (40) |
| Chloroquine | 21 (87.5) | 13 (52) | |
| Hydroxychloroquine | 18 (75) | 8 (32) | |
| Ivermectin | 6 (25) | 7 (28) | |
| O2/ventilatory support | Nothing | 0 | 18 (72) |
| Oxygen (mask or nasal cannula) | 2 (8.33) | 7 (28) | |
| High-flow cannula | 7 (29.17) | 0 | |
| Invasive mechanical ventilation | 15 (62.5) | 0 | |
| Extubation failure (n=15) | Yes | 2 (13.33) | NA |
| No | 8 (53.33) | NA | |
| NA (deceased) | 5 (33.33) | NA | |
| Vital outcome | Alive | 19 (79.17) | 25 (100) |
| Dead | 5 (20.83) | 0 | |
ICU: intensive care unit; NA: not applicable.
Source: Own elaboration.
Discussion
The present study describes the clinical characteristics of patients with COVID-19 who were treated at the emergency department of a quaternary care hospital in Cali, Colombia, during the first months of the pandemic (March and April 2020). For analysis purposes, the sample was divided into two groups: patients requiring admission to the ICU (n=24) and those not requiring admission to the ICU (n=25). It was found that there were no differences in terms of sex between the two groups, but there were significant differences regarding age (p=0.020), being higher in the ICU admission requirement group (58 vs. 49 years), which is similar to what has been reported in other studies.12-14,17However, it should be noted that the average age of the participants in the present study was 53 years, whereas similar studies describe cohorts with age over 60 years, which may be explained by the fact that they were conducted in countries with a higher proportion of elderly people.18-26
Regarding comorbidities, the present study found that the group of patients who required ICU treatment had a higher frequency of chronic diseases, with obesity (37.5%), arterial hypertension (54.2%) and diabetes (33.33%) being the most common. This finding is consistent with the reports of similar studies, which show that patients with COVID-19 and chronic diseases are at greater risk of adverse outcomes.17,18,27,28
It should be borne in mind that, at the time this study was conducted, the institution where the research was carried out did not routinely include a clinical severity scale score in the medical records of the patients, so this variable was not considered. In July and September 2020, the Colombian Ministry of Health29,30published guidelines recommending the use of the National Early Warning Score 2 (NEWS-2) to define the level of home care monitoring for patients with COVID-19, the criteria of the Community Acquired Pneumonia Severity Score (CRB-65) in the emergency department to establish the need for hospitalization or care at home, and the Criteria for Community Acquired Pneumonia Severity of the American Thoracic Society to define the need for admission to the ICU.
With respect to laboratory results, a higher total leukocyte and neutrophil count and a lower number of lymphocytes were observed in individuals who required ICU, a finding similar to that of the study by Cattelan et al.12 in Italy in 303 patients with COVID-19 who were divided into two groups (ICU admission vs. no ICU admission) in which these differences were maintained during follow-up examinations (p=0.055 at baseline count and p<0.01 at follow-ups).
Similarly, in a meta-analysis including 27 observational studies, Shi et al.3 1 reported that the pooled relative risk for COVID-19 mortality in patients with white blood cell count >10 000 was 6.41 (95%CI: 2.18-18.8). Leukocyte level elevation along with neutrophilia could indicate an additional infectious process; however, this scenario was not very likely in the present study since the values were obtained on admission when there is less chance of bacterial superinfection. Additionally, the leukocyte count was below 12 000 in most participants and severe bacterial infections usually show higher values.
The trend toward lymphopenia observed in the participants of the present study who required admission to the ICU (median=865/mm3) had already been reported by Chen et al.32 in a retrospective study in which they analyzed the clinical and immunological characteristics of 21 patients from Wuhan, China, with COVID-19. In that study, the authors found that CD4+ and CD8+ lymphocyte levels were lower in cases of severe COVID-19 than in those with moderate disease (severe vs. moderate cases: CD4+: 177.5 vs. 381.5 x 106/L; CD8+: 89.0 vs. 254.0 x 106/L). In this regard, it has been suggested that lymphopenia leads to a reduction in interferon-y production, which in turn decreases the response to virus infection. Recovery from this disorder has even been suggested as an important factor in the recovery process from COVID-19.10
The presence of neutrophilia and lymphopenia in patients requiring ICU admission found in the present study is consistent with reports in multiple studies that have established that an elevated neutrophil/lymphocyte ratio is predictive of COVID-19 severity.33-36For example, Sarkar et al.,35 in a meta-analysis conducted in 2022 that included 90 studies from Asia, Europe, and the United States, found that deceased and critically ill patients had an elevated baseline neutrophil/lymphocyte ratio on admission compared to survivors and non-critically ill patients (standardized mean: 3.82, 95%CI: 2.79-4.85 vs. 1.42, 95%CI: 1.22-1.63).
Another aspect to highlight in the present study is that CRP, ferritin and LDH levels were higher in patients who required intensive care (141.25/mgL vs. 27.95/mgL, 1 038 vs. 542.45, and 391 vs. 248.5, respectively), which is consistent with the results reported in other studies11,12,37,38in which higher values of this type of parameters have been reported in patients with worse outcomes.
Although CRP is a nonspecific inflammatory marker and, in some cases, may indicate bacterial superinfection, it is suggested that its early increase in patients with COVID-19 may be a severity marker that does not necessarily indicate concomitant bacterial infection. Similarly, elevated ferritin levels have been suggested as part of hemophagocytic lymphocytosis and cytokine storm syndrome in patients with severe COVID-19.10,39In turn, elevated LDH levels, recognized as a marker of tissue damage and a prognostic factor in several diseases, including interstitial lung disease,30,40have also been found to be associated with severity in COVID-19.31,41It should be noted that other markers not included in this study, such as D-dimer, have also shown prognostic value for COVID-19 severity.31,33,36,42
As for imaging characteristics, unilateral or bilateral presence of consolidation areas on chest x-ray was the only finding in which a significant difference was found between the two groups in the present study (p=0.005). Overall, ground-glass opacity was the most frequent feature (14.28%) following areas of consolidation (31.25%), but no significant difference was found between the two groups (p=0.246). In chest CT, the most common finding was ground-glass opacity (46.93%), which is in agreement with what has been reported in the literature.4,10,40,43
It has been determined that ground-glass opacities correspond to diffuse alveolar damage associated with the pathogenesis of viral infections.44 In this regard, Cocconcelli et al.13evaluated the extent of ground-glass lobar involvement and areas of consolidation on x-rays and assigned a score but found no association between the score and COVID-19 severity. Although the present study found significant differences in terms of the presence of consolidations between participants who required admission to the ICU and those who did not, the findings are not comparable with those of Cocconcelli et al.13 because of the difference in the methods of analysis used.
The most common complication found in the present study was bacterial superinfection in 37.50% of the patients admitted to the ICU. This may be related to the fact that the probabilities of developing bacterial superinfections when admitted to this service increase due to the presence of opportunistic microorganisms or because secondary infections are common in patients hospitalized for COVID-19, presenting in 10-30% of cases, with a much higher frequency in the ICU setting.45 Moola et al.46 established that bacterial coinfection was rare in patients with severe COVID-19 at the time of ICU admission, so it is reasonable to avoid early empirical antibiotic therapy. About this statement, in May 2021, Musuuza et al.47 published a meta-analysis that included 118 studies and in which they found that the pooled prevalence of global superinfection was 24% (95%CI: 19-30%) and that it was 41% (95%CI: 24-58%) in ICU patients; the latter figure was similar to the 37.50% found in the present study.
In their systematic review on imaging and clinical characteristics of patients with COVID-19 in which 31 articles with 46 959 patients in total were included, Cao et al.44 reported that 29.3% (95%CI: 0.190-0.395) of cases required ICU care and that the incidence of acute respiratory distress syndrome (ARDS), acute cardiac injury, acute renal failure, shock, and multiple organ dysfunction syndrome was 28.8% (95%CI: 0.147-0.429), 14.1% (95%CI: 0.079-0.204), 7.1% (95%CI: 0.031-0.110), 4.7% (0.009-0.086), and 8.5% (95%CI: -0.008-0.17), respectively. In the present study, kidney failure and bacterial superinfection were the most frequent complications in the group requiring ICU management: 5 patients progressed to multiple organ dysfunction and 4 of these died.
In a retrospective study, Ionescu et al.48 found that of 5 632 patients treated between March 12 and October 19, 2020, in 8 hospitals in southeastern Michigan (United States), 866 required IMV, of which they analyzed 281 and established an extubation (reintubation) failure rate of 33.1% (n=93). In the present study, the occurrence of this outcome was much lower (13.33%), which could be due to the fact that the institution where the research was conducted follows standardized protective ventilation protocols and uses the prone position in early stages to treat patients with acute respiratory distress syndrome (ARDS). In this regard, Liu et al.49 conducted a prospective multicenter observational study between August 31 and September 30, 2012, in 20 ICUs located in China, in which they found that mortality in patients with ARDS was 60%, which was largely attributed to the low level of positive expiratory pressure and to the scarce use of complementary treatments such as prone position and the use of neuromuscular blockers.
The good results found in the present study regarding mortality could also be explained by the use of high-flow cannulas in 29.17% of the patients admitted to the ICU in the early stages of the pandemic since, as reported by Bonnet et al.50 in a retrospective study involving patients with COVID-19 and acute respiratory failure hospitalized from March 11 to May 3, 2020, in 2 ICUs of tertiary care hospitals in Paris (France), patients who were treated with high-flow cannula required less IMV (OR=0.37, 95%CI: 0.18-0.76; p=0.007).
So far, there is no favorable evidence of the therapeutic efficacy of some medications used for the treatment of COVID-19, regardless of disease severity.51,52In the present study, the use of aminoquinolone derivatives was the most commonly used drug treatment in patients who required ICU management, whereas those who did not require ICU management were given both aminoquinolones and the combination lopinavir/ritonavir. These pharmacological treatments were generally used during the beginning of the pandemic, and although hydroxychloroquine has been confirmed to show antiviral effects in vitro, several controlled trials and meta-analyses have not revealed clinical benefits in the treatment of COVID-19 and, therefore, it is not recommended as a therapeutic option; moreover, the results of the studies for the lopinavir/ritonavir combination are also inconclusive and its use has not been associated with a reduction in mortality, hospital stay, or the need for IMV.53-55
The present study took into account not only clinical and demographic characteristics, but also included laboratory and imaging variables, which were compared depending on the need for ICU admission. Since this is a descriptive observational study, it has limitations for establishing associations and, given that only differences between known and observed factors were recorded, it is not possible to rule out the presence of other factors that explain the results and are beyond the scope of this analysis. In addition, due to the eligibility criteria of the study, the number of participants included does not allow performing the necessary analyses to make inferences of association or causality at the population level of the results obtained.
Since COVID-19 is an emerging infectious disease, sociodemographic, clinical and laboratory variables that determine the clinical outcomes of patients should be explored in depth, as it will allow the development of plans and strategies for prevention and early identification of individuals at greater risk of serious complications or even death. In this sense, it is necessary to carry out concurrent, analytical studies, with a larger sample size, to clarify the prognosis and treatment strategies of this disease.
Conclusions
Significant differences were observed in the values of several inflammatory markers, cellular damage, and hemogram parameters between patients who required admission to the ICU and those who did not, so these variables could be used to develop tools to help predict the prognosis of this disease.















