Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista Colombiana de Entomología
Print version ISSN 0120-0488
Rev. Colomb. Entomol. vol.38 no.2 Bogotá July/Dec. 2012
Immunological response of resistant and susceptible Plutella xylostella(Lepidoptera: Plutellidae) to Bacillus thuringiensis
Respuesta inmunológica de Plutella xylostella (Lepidoptera: Plutellidae) resistente y susceptible a Bacillus thuringiensis
Lílian Maria da Solidade Ribeiro1, Valéria Wanderley-Teixeira2, Franklin Magliano da Cunha1,2, Álvaro Aguiar Coelho Teixeira2,2 and Herbert Álvaro Abreu de Siqueira1,3
1 Universidade Federal Rural de Pernambuco, Departamento de Agronomia, Av. Dom Manoel de Medeiros s/n, Dois Irmãos CEP 52171-900, Recife, Pernambuco, Brazil. M. Sc., lilian_biology@yahoo.com.br, corresponding author.
1,2 D. Sc. ukento@yahoo.com.br
1,3 Prof. Dr., siqueira@depa.ufrpe.br
2 Universidade Federal Rural de Pernambuco, Departamento de Morfologia e Fisiologia Animal, Av. Dom Manoel de Medeiros s/n, Dois Irmãos CEP 52171- 900, Recife, Pernambuco, Brazil. Prof. Dra. valeria@dmfa.ufrpe.br
2,2 Prof. Dr. alvaro@dmfa.ufrpe.br
Received: 27-Dic-2011 - Accepted: 9-Oct-2012
Abstract: Despite the proven efficiency of Bacillus thuringiensis (Bt) to insects, a number of resistance cases have been reported throughout the world particularly for Plutella xylostella (Lepidoptera: Plutellidae) populations. Although the mechanism of resistance to Bt toxins due to altered receptors appears to be common among Lepidoptera, few studies have evaluated the immune response to Bt toxins in resistant insects. Thus, this study aimed to compare the total and differential counts of hemocytes as well as the nitric oxide levels in resistant and susceptible populations of P. xylostella to Bt after exposure to Dipel®PM (Btk) and Xentari®WDG (Bta) commercial formulations. Susceptible and resistant insects were exposed to their corresponding LC50 of Dipel®PM or Xentari obtained through dose-response curves. Hemolymph samples were collected at 1, 6, and 12 h after treatment for total and differential hemocyte counts and quantification of nitric oxide levels. Dipel®PM caused a significant reduction in the total number of hemocytes in the larvae of both populations, while Xentari®WDG affected only the proportion of types of hemocytes. Both formulations interfered with the nitric oxide levels, although no difference was observed between both populations.
Key words: Diamondback moth. Resistance. Humoral response. Hemocytes. Cellular response.
Resumen: A pesar de la probada eficacia de Bacillus thuringiensis (Bt) a los insectos, se han reportado ciertos casos de resistencia alrededor del mundo, especialmente para las poblaciones de Plutella xylostella (Lepidoptera: Plutellidae). Aunque el mecanismo de resistencia a las toxinas Bt, debido a la alteración de los receptores parece común entre los lepidópteros, pocos estudios han evaluado la respuesta inmune a las toxinas Bt en insectos resistentes. Así, este estudio tuvo como objetivo comparar el recuento total y diferencial de hemocitos tanto como los niveles de óxido nítrico en poblaciones de P. xylostella resistente y susceptible a Bt después de la exposición a las formulaciones comerciales de Dipel®PM (Btk) y Xentari®WDG (Bta). Insectos resistentes y susceptibles fueron expuestos a su CL50 correspondientes de Dipel or Xentari obtenidos a través de curvas dosis-respuesta. Muestras de hemolinfa fueron recogidas a las 1, 6 y 12 h después del tratamiento para el recuento total y diferencial de hemocitos y la cuantificación de los niveles de óxido nítrico. Dipel®PM causó una reducción significativa en el número total de hemocitos en las larvas de ambas poblaciones, mientras que Xentari afectó sólo la proporción de tipos de hemocitos. Ambas formulaciones interfirieron con los niveles de óxido nítrico, aunque no se observó diferencia entre las dos poblaciones.
Palabras clave: Polilla de las crucíferas. Resistencia. Respuesta humoral. Hemocitos. Respuesta celular.
Introduction
The diamondback moth, Plutella xylostella (L., 1758) (Lepidoptera: Plutellidae), is the major pest of Brassicaceae crops, demanding considerable expenditures on chemical and biological insecticides for its control (Talekar and Shelton 1993). The ease of evolving to resistance hinders the management of this moth, which has become resistant to at least 76 unique compounds (Whalon et al. 2008), including those products based on the Bacillus thuringiensis (Berliner, 1915) bacterium.
Reports of resistant populations of P. xylostella to B. thuringiensis have occurred in various parts of the world. The resistance was observed by Tabashnik et al. (1990), Zhao et al. (1993), Perez and Shelton (1997) and Wright et al. (1997) in USA (Florida, Hawaii and New York), Central American (Costa Rica, Guatemala, Honduras and Nicaragua) and Asian (Japan, Malaysia) populations. In Brazil, Castelo Branco et al. (2003) observed resistance of the pest populations from both environments where it was common to use B. thuringiensis and those where the entomopathogen was not used as insecticide.
Several mechanisms of resistance to B. thuringiensis (Bt) have been proposed for insects (Ferré and Van Rie 2002). The most well known involves changes in the binding receptors of the toxin in the columnar cells of the midgut, as reported in resistant strains of P. xylostella (Ferré and Van Rie 2002; Sayyed et al. 2004). However, low activation or high degradation of protoxins (Forcada et al. 1996), differential proteolytic activation (Lightwood et al. 2000), and sequestering by proteases (Milne et al. 1995) have also been implicated in the resistance. In addition, Rahman et al. (2004) showed a relation between the tolerance of the Ephestia kuehniella Zeller, 1879 (Lepidoptera: Pyralidae) with a high immune response and Ma et al. (2005) reported in Helicoverpa armigera (Hübner, 1805) (Lepidoptera: Noctuidae), an increase in melanization and coagulation reactions that are thought to impede the solubilization of Bt toxins.
Insects have an innate immune system that is highly adapted and effective against different microbial species at concentrations that are potentially fatal to vertebrates (Kavanagh and Reeves 2004). According to Ribeiro and Brehélin (2006), cellular and humoral components mediate the innate immunity when hemocytes perform cell reactions that involve phagocytosis, nodules formation, and encapsulation. The variable number and proportion of hemocytes in the hemolymph is one of the most important parameters during the infection process. At responding to pathogens, these variations not only result from the increase of some cells number, but also from hemocytes that migrate to the infection site and aggregate around the invader, and consequently forming nodules or capsules that immobilize the invader (Lavine and Strand 2002; Silva 2002). Humoral defense involves the action of antimicrobial peptides, a complex enzyme cascade that regulates hemolymph coagulation or melanization (Lavine and Strand 2002), oxygen-dependent mechanisms such as synthesis of lysozyme, superoxide dismutase-mediated synthesis of peroxides (Colinet et al. 2011; Nappi et al. 1995; Whitten et al. 2001) and synthesis of hypochlorite by myeloperoxidase (Kavanagh and Reeves, 2004), as well as the generation of intermediate reactive nitrogen (Tsakas and Marmaras, 2010).
Because changes in the immune response has been associated with tolerance of some species of insects to Bt, this study aimed to compare the differential and total count of hemocytes and nitric oxide levels in resistant and susceptible populations of P. xylostella after exposure to commercial formulations of Bt (Dipel (Btk) and Xentari (Bta)).
Material and Methods
Insects. Susceptible and resistant populations of P. xylostella to the commercial formulations Dipel®PM (B. thuringiensis var. kurstaki HD-1, containing Cry1Aa, Cry1Ab, Cry1Ac, Cry2Aa, and Cry2Ab) and Xentari®WDG (B. thuringiensis var. aizawai ATTC SD-1372, containing Cry1Aa, Cry1Ab, Cry1C, and Cry1D) were used in the study. The populations were collected from brassica crops in the municipalities of Chã Grande (Susceptible) and Camocin de São Félix (Resistant) from Pernambuco State, Brazil. The susceptible population has been reared under laboratory conditions without insecticide selection since 1998, whereas the resistant population was collected from an area with reported Xentari®WDG control failures and brought to the laboratory where they are raised on insecticide-free kale (Brassica oleracea var. acephala) leaves. The resistant population bred to F1 when it was used for the susceptibility tests and hemolymph collection.
Susceptibility bioassays. Concentration-response curves were established with the insecticides Dipel®PM and Xentari®WDG for both populations through bioassays to estimate LCs to be used in subsequent experiments. To bioassay the populations, 5 cm leaf discs of kale (sterilized with 5% sodium hypochlorite solution) were treated with increasing concentrations of each insecticide diluted with aqueous 0.01% Triton X-100 solution and left to dry at room temperature. The concentrations 0.062, 0.125, 0.25, 0.5, 1.0, 2.0, and 4.0 mg/L of (Dipel®WP) and 1.56, 3.12, 6.25, 12.5, 25, 50 and 100 mg/L (XenTari®WDG) were used against the susceptible population, while the concentrations 12.5, 25, 50, 100, 200, 400 and 800 mg/L (Dipel®WP) and 50, 100, 200, 400, 600, 800 and 1000 mg/L (XenTari®WDG) were used against the resistant population. The control comprised aqueous 0.01% Triton X-100 solution only. Both Petri dishes (60 x 15 mm) lined with water-moistened filter paper (5 cm) and treated leaf discs comprised the arenas to where 10 second-instar larvae were transferred. Each concentration was tested three times and the bioassay repeated at least once. The larvae were exposed for 72 hours in a growth chamber set to the following conditions: temperature of 25 ± 1 ºC, relative humidity of 60 ± 10%, and 12 hr photoperiod. Mortality was assessed using the criterion of no larval movement after touching larvae with a fine brush.
Hemocytes characterization, total and differential counts. Fourth-instar larvae of both populations were subjected to LC50 of both Dipel®PM and Xentari®WDG, comparing with the control treatment (larvae fed with leaves treated with distilled water plus Triton X-100 at 0.01%) and further used for counts. The morphological characterization of hemocytes was performed using as reference the hemocytes from larvae of the control treatment. Larvae were exposed to insecticide-treated kale leaf discs (70 mm in diameter, 30 sec immersion) after drying at room temperature and transferred into Petri dishes (80 x 15 mm) containing water-moistened filter paper (70 mm in diameter). Hemolymph samples were collected 1, 6, and 12 h following treatment. For total hemocyte counts, 0.5 µL aliquot of hemolymph from two larvae (1 µL total) was collected through an incision made between the larvae pseudo legs. The aliquots were diluted in 9.0 µL of anticoagulant II solution for insects (Mead et al. 1986). The counting took place with the aid of an optical microscope after overlaying the samples in a Neubauer chamber. The perfusion method described by Brayner et al. (2005) determined the differential counting. The insect anticoagulant solution was injected into the larvae with the aid of a capillary glass with subsequent hemolymph suction and transfer to a glass slide. Each slide containing the hemolymph of one larva represented a replicate for each time interval/treatment and repeated ten times. The slides were dried at room temperature, stained with Giemsa, and examined under an optical microscope. Hemocyte dynamics and possible morphological alterations analysis in the cell types comprised 300 cells per slide for all treatments (Falleiros et al. 2003).
Nitric oxide levels in the hemolymph. Nitric oxide levels were determined using the Griess reagent (Green et al. 1981) by the method described by Faraldo et al. (2005). Nitrite (NO2-) titration in the hemolymph following treatment with the insecticides was compared among the samples collected at 1, 6, and 12 h. Each hemolymph sample comprised ten 4th-instar larvae, totaling 5 µL, and transferred to Eppendorf tubes containing 55 µL of sulfanilamide (1%) in H3PO4 (5%). The samples were stored at -20 ºC until use. To determine the concentration of NO2-, aliquots of 30 µL of each sample/time/treatment (five samples) were pipetted in triplicate into a 96-well flat-bottom plate containing 30 µL of naphthylenediamine dihydrochloride (NEED, Sigma, St. Louis, MO). Absorbance was measured after five minutes with the aid of a microplate reader using a 562 nm filter and a NaNO2 standard curve estimated the NO2- quantities.
Statistics analysis. Mortality data were analyzed through Probit analysis (Finney 1971) after correction by the mortality in the control (Abbott 1925) using the POLO-Plus program (LeOra-Software 2005). Lethal concentration (LC50) values were estimated and the resistance ratios between the populations calculated by “lethal ratio test” according to Robertson and Preisler (1992). The data from total and differential counts as well as the nitric oxide titration were submitted to ANOVA using a 3 x 2 x 3 factor scheme, considering the three treatments (Control, Dipel®PM, and Xentari®WDG), two populations (susceptible and resistant), and three evaluation times (1, 6, and 12 h). Tukey's HSD test (P > 0.05) compared the mean values and, when necessary, the Student's T-test was used. Following the normality test, data on the total count were log transformed (x + 1), whereas the data on the differential count (only for the analysis of prohemocytes, spherulocytes, adipohemocytes, and oenocytoids) and nitric oxide titration were root transformed (x + 0.5). The SAS Statistical System (SAS Institute Inc. 1999) was used to perform the data analysis.
Results
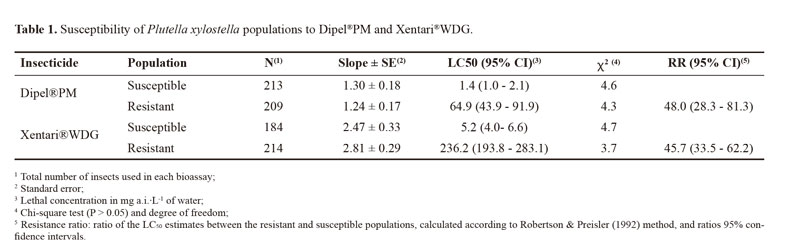
The estimated LC50 values for the susceptible population were respectively 1.4 and 5.2 mg a.i./L for Dipel®PM and Xentari®WDG, whereas the values for the resistant population were 64.9 and 236.2 mg a.i./L for Dipel®PM and Xentari®WDG, respectively. The Camocin of São Félix population was respectively 48.0- and 45.7-fold more resistant to Dipel®PM and Xentari®WDG in comparison to the susceptible population (Table 1).
The total number of circulating hemocytes in the hemolymph of fourth-instar P. xylostella larvae was significantly lower in the resistant population than in the susceptible population (Fig. 1). However, insects from both populations exhibited a significant reduction in the total number of hemocytes following exposure to Dipel®PM, whereas no significant change in hemocyte number was observed in the insects challenged with Xentari®WDG (Fig. 1). No differences were found between the time intervals analyzed (F = 2.57; DF = 2; P = 0.0795).
Morphological analysis revealed the existence of six cell types in the hemolymph of P. xylostella: plasmatocytes, granulocytes, prohemocytes, spherulocytes, adipohemocytes, and oenocytoids. No morphological alterations were found in these cell types following treatment with Dipel®PM or Xentari®WDG, neither in the susceptible nor in the resistant population. The most abundant cell types in the hemolymph of the resistant population were plasmatocytes (84.01%), followed by granulocytes (9.2%), spherulocytes (3.03%), oenocytoids (2.4%), adipohemocytes (0.76%), and prohemocytes (0.6%). In the susceptible population, the most abundant cell types were plasmatocytes (81.53%), followed by granulocytes (12.86%), oenocytoids (2.21%), adipohemocytes (1.8%), spherulocytes (0.98%), and prohemocytes (0.62%). The amount of all cell types significantly differed between the populations except oenocytoids (F = 0.01; DF = 1; P = 0.9030). The resistant insects produced greater amounts of plasmatocytes, prohemocytes and spherulocytes than susceptible ones, whereas the latter exhibited a greater number of granulocytes and adipohemocytes (Fig. 2).
There was no population x treatment interaction among plasmatocytes (F = 0.25; DF = 2; P = 0.7808), granulocytes (F = 0.49; DF = 2; P = 0.6150), prohemocytes (F = 1.80; DF = 2; P = 0.1684), and oenocytoids (F = 1.37; DF = 2; P = 0.2575). Thus, the data of each population were pooled for the determination of the quantitative variation in these cell types with regarding to exposure to the insecticides. In the insects treated with Xentari®WDG, the number of plasmatocytes increased significantly, whereas the number of granulocytes, prohemocytes, and oenocytoids reduced significantly in relation to the control. Regarding the Dipel®PM treatment, only the number of oenocytoids was significantly different in relation to the control (Fig. 3).
Spherulocytes and adipohemocytes were the only cell types showing significant differences between populations after treatment with the insecticides. The number of spherulocytes was significantly higher in the control and insects treated with Xentari®WDG in the resistant population in comparison to the susceptible population (Fig. 4A).
The susceptible insects showed a lower number of these cells when treated with Xentari®WDG in comparison to Dipel®PM, although not differing significantly from the control. In the resistant insects, the number of spherulocytes was significantly lower following treatment with Xentari®WDG in comparison to the control (Fig. 4A).
The susceptible population showed a significantly higher number of adipohemocytes in the control and insects treated with Dipel®PM than the resistant population (Fig. 4B). In the resistant insects, the number of these cells did not differ significantly between treatments, whereas a significantly lower number of adipohemocytes existed following the treatment with Xentari®WDG in the susceptible insects (Fig. 4B).There were significant differences between evaluation times only for prohemocytes and adipohemocytes, which significantly reduced at 6 hours (F = 3.18; DF = 2; P = 0.0440 and F = 3.55; DF = 2; P = 0.0307, respectively). In the statistical analysis of the interaction between treatments and evaluation times, there was an accentuated reduction in adipohemocytes in comparison to the control only at the 1-h evaluation time, which was significant for Xentari®WDG (Table 2).
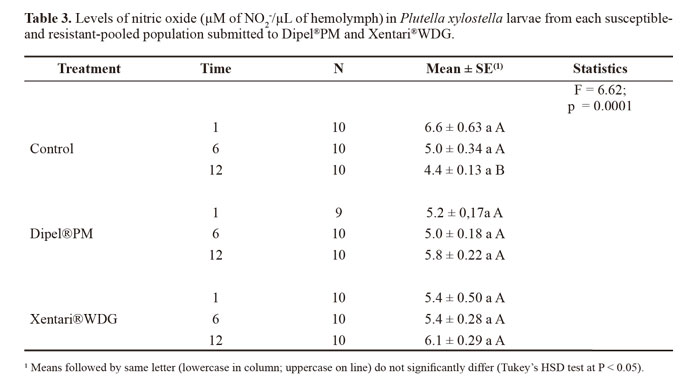
Nitric oxide levels did not significantly differ between populations (F = 0.81; DF = 2; P = 0.4469). Thus, the data from each population were pooled for the analysis of the effects of time and treatment. The concentration of nitric oxide increased in the insects treated with the insecticides in comparison to the control, with the highest increase at 12 h in both treatments (Table 3).
Discussion
The morphological analysis revealed six cell types in the hemolymph of P. xylostella, which followed the morphological pattern described by other authors for the order Lepidoptera (Falleiros et al. 2003; Negreiro et al. 2004; Correia et al. 2008). The total count revealed a significantly lower number of circulating hemocytes in the Bt resistant larvae, which was also observed by Ericsson et al. (2009) for a Bt resistant strain of Trichoplusia ni (Hubner) (Lepidoptera: Noctuidae), suggesting a possible fitness cost associated with resistance or that reduced binding of toxin to receptors does not induce the immune response to the same level as the susceptible colony (Ericsson et al 2009). Despite this, the differential count showed that the proportion of plasmatocytes, prohemocytes, and spherulocytes was higher in the resistant population in comparison to the susceptible population. These cells are directly involved in the cell defense response (Lavine and Strand 2002; Costa et al. 2005; Ling et al. 2005; Ling and Yu 2006). As the success of the response depends not only on the number but also on the types of hemocytes involved (Russo et al. 2001), this could be a result of a compensatory mechanism to meet the reduction of the total number of hemocytes in the resistant population.
Exposure of both P. xylostella populations to the Dipel®PM Bt formulation significantly reduced the total number of hemocytes, suggesting that Bt-based formulations may differently affect the immune response regardless of pest susceptibility. The reduction in hemocytes may have stemmed from an increase in cell activity as demonstrated by Dubovskiy et al. (2008) who found an increase in phagocyte activity and encapsulation rate in Galleria mellonella (L., 1758) (Lepidoptera: Pyralidae) larvae challenged with sub-lethal doses of B. thuringiensis. Mostafa et al. (2005) also found an increase in nodules formation in the hemocoel of E. kuehniella (Lepidoptera: Pyralidae) larvae fed with wheat containing B. thuringiensis var. kurstaki. Although the treatment with Xentari®WDG did not cause a significant change in the total number of hemocytes, it caused changes in the number of different cell types in the hemolymph in both P. xylostella populations, whereas Dipel®PM caused significant reduction only in the number of oenocytoids. This change in the numbers of plasmatocytes and granulocytes is likely associated with the function of these cell types, which are directly involved in the cell defense process through phagocytosis and the formation of nodules (Lavine and Strand 2002). A reduction in granulocyte may be due to their involvement in the initial nodulation process, since they are the first cells to recognize exogenous agents (Dean et al. 2004), whereas plasmatocytes only participate in the final stages of this process, which could explain the increase in their number. The reduction in prohemocytes and increase in plasmatocytes suggest that prohemocytes, which are undifferentiated cells, differentiated into plasmatocytes, thereby increasing the pool of plasmatocytes (Jiravanichpaisal et al. 2006). This process seemed to be more intense in resistant insects, which had a higher number of prohemocytes and plasmatocytes circulating in the hemolymph.
Nodule formation is generally followed by the melanization process, which results in oxidation reactions catalyzed by the enzyme phenoloxidase, therefore the lysis of oenocytoids for the release of this enzyme would explain their reduced number in the hemolymph (Lavine and Strand 2002; Silva 2002).
The reduction of adipohemocytes in susceptible insects treated with Xentari®WDG may be associated with the consumption of energy reserves of these cells in order to repair the damage caused by the Cry toxin in the larvae midgut (Hillyer and Christensen 2002).
The number of spherulocytes was significantly higher in the control and Xentari®WDG treatments in the resistant population, being the third most frequent cell type in the hemolymph of these individuals when compared with the susceptible population. The reasons why the resistant population showed a high number of spherulocytes in the control treatment is not clear, but it may reflect the immune status of this population. This immune response in the resistant insects after treatment with Xentari®WDG possibly provides an advantage over susceptible larvae, since these cell types play a role in the defense against bacteria. The spherulocytes mediate the production and release of heteroagglutinins, which act in the destruction of these microorganisms (Yeaton 1983).The insects treated with the insecticides showed a higher titration in relation to the control only at 12 h of exposure, perhaps indicating the involvement of nitric oxide during the immune defense process in P. xylostella. The increase in nitric oxide titration has been reported in insects challenged by pathogens and parasitoids (Nappi et al. 2000; Faraldo et al. 2005).
Dipel®PM proved to be effective at reducing the total number of hemocytes in the larvae of both resistant and susceptible populations, while Xentari®WDG affects only the differential count of these cells. Both formulations interfered with the nitric oxide levels, although no difference was observed between both populations.
Acknowledgments
The authors thanks the Fundação de Apoio a Pesquisa do Estado de Pernambuco (FACEPE) for the first author support through a M.Sc. assistantship.
Literature cited
Abbott, W.S. 1925. A method of computing the effectiveness of an insecticide. Journal Economic Entomology 18: 265-267. [ Links ]
Brayner, F. A.; Araújo, H. R. C.; Cavalcanti, M. G. S.; Alves, L. C.; Peixoto, C. A. 2005. Ultrastructural characterization of the hemocytes of Culex quinquefasciatus (Diptera: Culicidae). Micron 36 (4): 359-367. [ Links ]
Castelo Branco, M.; França, F. H.; Pontes, L. A.; Amaral, P. S. T. 2003. Avaliação da suscetibilidade a inseticidas em populações da traça-das-crucíferas de algumas áreas do Brasil. Horticultura Brasileira 21 (3): 549-552. [ Links ]
COLINET, D.; CAZES, D.; BELGHAZI, M.; GATTI, J.-L.; POIRIÉ, M. 2011. Extracellular superoxide dismutase in insects characterization, function, and interspecific variation in parasitoid wasp venom. Journal of Biological Chemistry 286 (46): 40111-40121. [ Links ]
Correia, A. A.; Wanderley-Teixeira, V.; Oliveira, J. V.; Torres, J. B. 2008. Dinámica hemocitaria en larvas de Spodoptera frugiperda (J.E. Smith) (Lepidoptera: Noctuidae) tratadas con nim (Azadirachta indica A. Juss). Boletín de Sanidad Vegetal Plagas 34: 357-365. [ Links ]
Costa, S. C. P.; Ribeiro, C.; Girard, P. -A.; Zumbihl, R.; Brehélin, M. 2005. Modes of phagocytosis of Gram-positive and Gram-negative bacteria by Spodoptera littoralis granular haemocytes. Journal of Insect Physiology 51 (1): 39-46. [ Links ]
Dean, P.; Potter, U.; Richards, E. H., Edwards, J. P., Charnley, A. K., Reynolds, S. E. 2004. Hyperphagocytic haemocytes in Manduca sexta. Journal of Insect Physiology 50 (11): 1027-1036. [ Links ]
Dubovskiy, I. M.; Krukova, N. A.; Glupov, V. V. 2008. Phagocytic activity and encapsulation rate of Galleria mellonella larval haemocytes during bacterial infection by Bacillus thuringiensis. Journal of Invertebrate Pathology 98 (3): 360-362. [ Links ]
Ericsson, J. D.; Janmaat, A. F.; Lowenberger, C.; Myers, J.H. 2009. Is decreased generalized immunity a cost of Bt resistance in cabbage loopers Trichoplusia ni? Journal of Invertebrate Pathology 100 (2): 61-67. [ Links ]
Falleiros, Â. M. F.; Bombonato, M. T. S.; Gregório, E. A. 2003. Ultrastructural and quantitative studies of hemocytes in the sugarcane borer, Diatraea saccharalis (Lepidoptera: Pyralidae). Brazilian Archives of Biology and Technology 46 (2): 287-294. [ Links ]
Faraldo, A. C.; Sá-Nunes, A.; Del Bel, E. A.; Faccioli, L. H.; Lello, E. 2005. Nitric oxide production in blowfly hemolymph after yeast inoculation. Nitric Oxide 13 (4): 240-246. [ Links ]
Ferré, J.; Van Rie, J. 2002. Biochemistry and genetics of insect resistance to Bacillus thuringiensis. Annual Review of Entomology 47: 501-533. [ Links ]
Finney, D. J. 1971. Probit analysis. Cambridge University Press, London. 68 p. [ Links ]
Forcada, C.; Alcácer, E.; Garcerá, M. D.; Martínez, R. 1996. Differences in the midgut proteolytic activity of two Heliothis virescens strains, one susceptible and one resistant to Bacillus thuringiensis toxins. Archives of Insect Biochemistry and Physiology 31 (3): 257-272. [ Links ]
Green, L. C.; Ruiz de Luzuriaga, K.; Wagner, D. A.; Rand, W.; Istfan, N.; Young, V. R.; Tannenbaum, S. R. 1981. Nitrate biosynthesis in man. Proceedings of the National Academy of Sciences of the United States of America 78: (12) 7764-7768. [ Links ]
Hillyer, J.; Christensen, B. 2002. Characterization of hemocytes from the yellow fever mosquito, Aedes aegypti. Histochemistry and Cell Biology 117 (5): 431-440. [ Links ]
Jiravanichpaisal, P.; Lee, B. L.; Söderhäll, K. 2006. Cell-mediated immunity in arthropods: Hematopoiesis, coagulation, melanization and opsonization. Immunobiology 211 (4): 213-236. [ Links ]
Kavanagh, K.; Reeves, E. P. 2004. Exploiting the potential of insects for in vivo pathogenicity testing of microbial pathogens. FEMS Microbiology Reviews 28: 101-112. [ Links ]
Lavine, M. D.; Strand, M. R. 2002. Insect hemocytes and their role in immunity. Insect Biochemistry and Molecular Biology 32 (10): 1295-1309. [ Links ]
LeOra-Software, 2005. POLO-Plus, POLO for Windows. Petaluma, LeOra Software. [ Links ]
Lightwood, D.J.; Ellar, D.J.; Jarrett, P. 2000. Role of Proteolysis in Determining Potency of Bacillus thuringiensis Cry1Ac delta-Endotoxin. Applied and Environmental Microbiology 66 (12): 5174-5181. [ Links ]
Ling, E.; Shirai, K.; Kanekatsu, R.; Kiguchi, K. 2005. Hemocyte differentiation in the hematopoietic organs of the silkworm, Bombyx mori: prohemocytes have the function of phagocytosis. Cell and Tissue Research 320 (3): 535-543. [ Links ]
Ling, E.; Yu, X. -Q. 2006. Hemocytes from the tobacco hornworm Manduca sexta have distinct functions in phagocytosis of foreign particles and self dead cells. Developmental & Comparative Immunology 30 (3): 301-309. [ Links ]
Ma, G.; Roberts, H.; Sarjan, M.; Featherstone, N.; Lahnstein, J.; Akhurst, R.; Schmidt, O. 2005. Is the mature endotoxin Cry1Ac from Bacillus thuringiensis inactivated by a coagulation reaction in the gut lumen of resistant Helicoverpa armigera larvae? Insect Biochemistry and Molecular Biology 35 (7): 729-739. [ Links ]
Mead, G. P.; Ratcliffe, N. A.; Renwrantz, L. R. 1986. The separation of insect haemocyte types on Percoll gradients: methodology and problems. Journal of Insect Physiology 32 (2): 167-177. [ Links ]
Milne, R. E.; Pang, A. S. D.; Kaplan, H. 1995. A protein complex from Choristoneura fumiferana gut-juice involved in the precipitation of [delta-endotoxin from Bacillus thuringiensis subsp, sotto. Insect Biochemistry and Molecular Biology 25 (10): 1101-1114. [ Links ]
Mostafa, A. M.; Fields, P. G.; Holliday, N. J. 2005. Effect of temperature and relative humidity on the cellular defense response of Ephestia kuehniella larvae fed Bacillus thuringiensis. Journal of Invertebrate Pathology 90 (2): 79-84. [ Links ]
Nappi, A. J.; Vass, E.; Frey, F.; Carton, Y. 1995. Superoxide anion generation in Drosophila during melanotic encapsulation of parasites. European Journal of Cell Biology 68 (4): 450-456. [ Links ]
Nappi, A. J.; Vass, E.; Frey, F.; Carton, Y. 2000. Nitric oxide involvement in Drosophila immunity. Nitric Oxide 4 (4): 423-430. [ Links ]
Negreiro, M. C. C.; Andrade, F. G. D.; Falleiros, Â. M. F. 2004. Sistema imunológico de defesa em insetos: uma abordagem em lagartas da soja, Anticarsia gemmatalis Hübner (Lepidoptera: Noctuidae), resistentes ao AgMNPV. Semina: Ciências Agrárias 25 (4): 293-308. [ Links ]
PÉrez, C. J.; Shelton, A. M. 1997. Resistance of Plutella xylostella (Lepidoptera: Plutellidae) to Bacillus thuringiensis Berliner in Central America. Journal of Economic Entomology 90 (1): 87-93. [ Links ]
Rahman, M. M., Roberts, H. L. S., Sarjan, M., Asgari, S., Schmidt, O. 2004. Induction and transmission of Bacillus thuringiensis tolerance in the flour moth Ephestia kuehniella. . Proceedings of the National Academy of Sciences of the United States of America 101 (9): 2696-2699. [ Links ]
Ribeiro, C.; Brehélin, M. 2006. Insect haemocytes: What type of cell is that? Journal of Insect Physiology 52 (5): 417-429. [ Links ]
Robertson, J. L.; Preisler, H. K. 1992. Pesticide bioassays with arthropods. CRC Press, Inc., Boca Raton. 125 p. [ Links ]
Russo, J.; Brehélin, M.; Carton, Y. 2001. Haemocyte changes in resistant and susceptible strains of D. melanogaster caused by virulent and avirulent strains of the parasitic wasp Leptopilina boulardi. Journal of Insect Physiology 47 (2): 167-172. [ Links ]
SAS Institute Inc., 1999. STAT User's guide. SAS Institute, Cary. [ Links ]
Sayyed, A. H.; Raymond, B.; Ibiza-Palacios, M. S.; Escriche, B.; Wright, D.J. 2004. Genetic and biochemical characterization of field-evolved resistance to Bacillus thuringiensis toxin Cry1Ac in the diamondback moth, Plutella xylostella. Applied and Environmental Microbiology 70 (12): 7010-7017. [ Links ]
Silva, C. C. A. 2002. Aspectos do sistema imunológico dos insetos. Biotecnologia Ciência & Desenvolvimento 24: 68-72. [ Links ]
Tabashnik, B. E.; Cushing, N. L.; Finson, N.; Johnson, M.W. 1990. Field development of resistance to Bacillus thuringiensis in diamondback moth (Lepidoptera: Plutellidae). Journal of Economic Entomology 83 (5): 1671-1676. [ Links ]
Talekar, N. S.; Shelton, A. M. 1993. Biology, ecology, and management of the diamondback moth. Annual Review of Entomology 38: 275-301. [ Links ]
TSAKAS, S.; MARMARAS, V. J. 2010. Insect immunity and its signalling: an overview. Invertebrate Survival Journal 7(2): 228-238. [ Links ]
Whalon, M. E.; Mota-Sanchez, D.; Hollingworth, R. M. 2008. Analysis of global pesticide resistance in arthropods. p. 5-31. In: Whalon, M. E. (Ed.). Global Pesticide Resistance in Arthropods. CABI Publishing. Wallingford. United States of America. 192 p. [ Links ]
Whitten, M. M. A.; Mello, C.B.; Gomes, S.A.O.; Nigam, Y.; Azambuja, P.; Garcia, E.S.; Ratcliffe, N.A. 2001. Role of superoxide and reactive nitrogen intermediates in Rhodnius prolixus (Reduviidae)/Trypanosoma rangeli interactions. Experimental Parasitology 98 (1): 44-57. [ Links ]
Wright, D. J.; Iqbal, M.; Granero, F.; Ferré, J. 1997. A change in a single midgut receptor in the diamondback moth (Plutella xylostella) is only part responsible for field resistance to Bacillus thuringiensis subsp. kurstaki and Bacillus thuringiensis subsp. aizawai. Applied and Evironmental Microbiology 63 (5): 1814-1819. [ Links ]
Yeaton, R. W. 1983. Wound Responses in Insects. American Zoologist 23 (1): 195-203. [ Links ]
Zhao, J. Z.; Zhu, G. R.; Zhu Z. L.; Wang W. Z. 1993. Resistance of diamondback moth to Bacillus thuringiensis in China. Resistance Pest Management 5: 11-12. [ Links ]