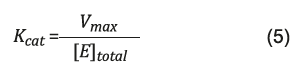Introduction
Enzymes are a group of organic substances with protein nature and intracellular or extracellular activity. Its primary function in organisms is to provide a reactive route of lower activation energy with a shorter reaction time and in mild conditions of temperature and pressure. Enzymes convert one substance called the substrate to a product, and they tend to be quite specific 1), (2), (3), (4. In biological processes, enzymes are preferable to chemical catalysts because they are more selective, resulting in cleaner product and a higher yield 5. However, the difficulty in recovering the enzyme from the reaction medium at the end of reaction, together with the instability in certain solvents and/ or conditions of pH, temperature and exposure to denaturing agents, makes them unsuitable for use in several processes. Enzymatic immobilization has emerged as an alternative to overcome these throwbacks. Enzyme immobilization has been shown to be a powerful tool to improve almost all enzymatic properties, such as high activity and selectivity 6. Immobilization is a process that can be defined as the attachment of enzymes or living cells to a surface, so that its catalytic activity is not adversely affected 7. The use in continuous process, the increase of the stability and the reutilization of the biocatalyst are the main advantages afforded by the immobilization 8. Research on substrates for immobilization of enzymes has been increasing. Various organic or inorganic materials may be used as supports for immobilization and during a selection for a given immobilization process the main properties to be analyzed are: surface area, insolubility, regenerability, reuse, resistance to microbial attack, mechanical resistance, contain a large number of sites to immobilize the enzyme and impose the least amount of limitations for the occurrence of reaction 9. However, the choice of support for immobilization is not only related to its chemical and physical properties, but also the characteristics of the enzyme to be immobilized and the process in which it will be applied. In the search for new materials capable to work as support for enzyme immobilization, carbon based materials have been increase in the last years. Biochar is a carbon material that have gain attention.
The term biochar is used for the solid fraction obtained from carbonized lignocellulosic materials during pyrolysis. It is produced for a variety of purposes, for example carrier of enzymes because of their porous characteristics. The characteristics of biochar depend on the raw material and the production conditions. In general, the biochar presents a carbonaceous molecular structure formed by piles of flat sheets of the aromatic rings randomly connected, and can assume acidic/basic, hydrophobic/hydrophilic behavior. The biochar is obtained by pyrolysis. The process consists of four different and well defined stages: drying of the material, decomposition of the hemicellulose, decomposition of the cellulose and, finally, the decomposition of the lignin 10.
Cassava is a perennial bush of intense production in tropical countries, belongs to the class of Dicotyledons, order Euphorbiales, family Euphorbiaceae and genus Manihot. The genus Manihot is composed of 98 species, the species ManihotesculentaCrantz is the only species cultivated commercially for the production of tuberous roots rich in starch 11.
The response of enzymes to biochar addition was dependent on the conditions of synthesis, application rate, soil texture and even the enzyme assayed 12. The long-term inappropriate utilization and management of soils can cause severe degradation, a study reporting a poor red soil incremented with biochar from oak and bamboo, evaluated the activity of enzymes and their effects on soil remediation. Dehydrogenase, β-glucosidase and urease had improved the soil quality due their interactions with biochar 13), (14. Soil extracellular enzymes involved in carbon and sulfur cycling indicated that amendment with the lower amount of maize biochar could increase soil enzyme activities, on the other hand, amendment with the higher amount reduces their activities 15. The activities of L-leucine aminopeptidase and urease further confirmed that adding biochar to soil could increase the activities of a series of enzymes related to N utilization 15.
Lipases (E.C.3.1.1.3) have been defined as carboxylesterases which hydrolyze long chain acylglycerols, i.e. with acyl chain consisting of more than 10 carbon atoms. Its natural substrates are oils and fats containing triacylglycerols made up of long chain fatty acids, i.e., triple ester bonds. In general, lipases do not require cofactors, act in a wide pH range, are stable at high temperatures; have high specificity and regio properties, chemo and enantio selectivity that make them highly applicable in industrial processes. The study of the enzymatic kinetics involves indirect information on the mechanism of catalytic action, specificity of the enzymes, factors that affect the speed of reactions and the quantitative determination of their effects. Although they catalyze a wide variety of reactions by different mechanisms, the enzymes can be analyzed for their velocities to quantify their efficiencies. When the substrate [S] binds to the active site of an enzyme [E], an enzyme-substrate complex [ES] is formed in a rapid and reversible process before the formation of the product [P]. After a short time, the product dissolves from the enzyme according to the mechanism below 16), (17), (18.
According to Michaelis-Menten, it is possible to know if an enzyme has affinity for the substrate by calculating the Km constant, also known as Michaelis-Menten constant. K m and demonstrates the binding strength of a substrate to an enzyme. Low values of K m reflect a high affinity of the enzyme for the substrate, allowing high reaction rates, even with low substrate concentrations 19. This work had the initial idea to investigate the affinity between the enzyme lipase and biochar produced from pyrolyzed cassava stump. The pyrolysis temperature was defined as 600 oC due to preliminary tests conducted by the research group 20. Since the aim is to immobilize the enzyme in the support, it is believed that the larger pore size induces to a higher amount of immobilized enzyme.
Materials and methods
Enzymatic immobilization
The enzyme used was porcine pancreas lipase, type II, code l3126, Sigma Aldrich; The enzyme activity indicated on the label by the manufacturer was 50 U.mg-1, where U is defined as 1 micromole of hydrolyzed sucrose per minute at pH 4.5 and 55 °C. This enzyme was selected because its diameter (3.5 nm) and molecular weight (135,000 Da) are suitable to allow its incorporation into the pores of the biochar.
Lipase was immobilized by adsorption. For this, 10 g of the support and 50 mL of enzymatic solution at different concentrations (0.1, 0.25 and 0.5 g/L) in 0.1 M phosphate buffer, pH 7, was placed in a beaker. The contact time was 24 hours at 25 °C. It was then sent to centrifugation at 11.000 x g for 20 min at 25 °C (Jouan Centrifuge, model BR4i, France). The blank, using 0.1 M phosphate buffer, pH 7.5, was prepared under the same conditions of the enzyme solutions.
After centrifugation, the enzymes were filtered on quantitative vacuum filter paper 11 µm (Aspirator Diapump, Fanem, model 089-A, São Paulo, Brazil). With the retained was determined the enzymatic activity and with the permeated, the amount of proteins was established.
Determination of enzymatic activity
Enzymatic activity was determined in 125 mL erlenmeyer flasks with 5 mL of emulsion prepared with 75% gum arabic and 25% extra virgin olive oil. 2 ml of 0.1 M phosphate buffer pH 7.0 was added; 1 mL of enzyme solution was added and the flasks were incubated at 37 ° C for 30 minutes in a thermostated bath with shaking of 130rpm. The reaction was stopped by addition of 15 mL of 1:1 acetone:ethanol and the liberated fatty acids were titrated against 0.05 N NaOH solution using phenolphthalein as indicator.
The enzymatic activity (A enz. ) of lipases were determined by equation 2 23:
V a = volume of sample titrated (mL)
V b = titrated volume of white (mL) N = Normality of the NaOH solution t = Reaction time (min) m = Mass of the sample used in the reaction (g)
Determination of protein concentration (Qp) The protein dosage was determined by Bradford method (1976). This method consists of dissolving 50 mg of Coomassie G brilliant blue(Sigma) in 25 mL of ethanol under constant stirring. Then, 50 mL of 85% phosphoric acid and 500 mL of distilled water are added. The solution should be kept under stirring for 1 hour followed by filtration with the filter described previously 21.
Before determining the amount of protein in the samples, it is necessary to construct a calibration curve that serves as the basis for calculations of protein quantification. The curve used was the standard curve with different concentrations of bovine serum albumin (BSA) in presence of dye solution. This curve was constructed using BSA solutions at the range 0.02 g/L to 0.10 g/L. The points of the curve were determined using mixtures containing 0.5 mL aliquots of BSA solutions and 5 mL of dye solution. After 10 minutes in contact, the absorbance was read in a spectrophotometer at 600 nm, taking as zero the absorbance of a sample containing 0.5 mL of water and 5 mL of dye solution. The protein concentration in the samples was easily found by replacing the absorbance values in the equation of the line of the albumin curve.
Immobilization yield
From the values of enzymatic activity, it was possible to determine the percentage amount of enzyme immobilized in the support (yield of immobilization), using the following equation (3) 22:
Where:
R(%) = Immobilization yield;
A i = Enzymatic activity of the free enzyme
(enzymatic solution);
A f = Enzyme activity of the immobilized enzyme.
Study of the lipase affinity
In the evaluation of the lipase immobilized affinity under the biochar, an alternative approach was adopted using the classic Michaelis-Menten model (Equation 4).
The parameter value K m indicates the substrate concentration [S] required to obtain an initial velocity (ν 0 ) which is half the maximum velocity (V max ) 23.
The experimental results of enzyme activity versus enzyme concentration in the immobilization solution were adjusted to the Michaelis-Menten model with the aid of Statistic® 11.0 software, using the Levenberg-Marquardt numerical method 24 to determine the values of V max and K m of each biocatalyst. The catalytic efficiency of the enzyme was determined by the catalytic constant, K cat , through equation 5 23.
From the K cat and K m values it was possible to evaluate if the support showed interaction with the enzyme lipase. [E] total means enzyme concentration.
Results and discussion
Table 1 shows the results of enzymatic activity (Aenz), amount of protein (Qp) and immobilization yield (R) obtained for the pyrolyzed biochar at 600 °C to different enzymatic solutions.
It is possible to notice that the lipase, independent of the concentration of the enzymatic solution, was able to adhere to the biochar. A high yield of immobilization (61.2%) was found when a higher amount of enzymes was used, 0.5 g/L. From this point, it was a satisfactory yield, especially when comparing with the binding efficiency of lipase in the range between 40-60%, using biochar produced from oat hull pyrolysis at 300 C° and depending on biochar particle size 25. Ribeiro and co-workers 4, high yields of invertase enzyme immobilization ranging from 50.1 to 84.5 for enzyme concentrations of 0.5 and 0.1 g L−1, respectively. This result indicates that more than half of the catalytic activity of the free enzyme was retained after the immobilization process, showing that the adopted procedure was appropriate because it does not significantly alter the biocatalyst efficiency compared with other modification processes.
Table 1 Enzymatic activity and immobilization efficiency of immobilized enzyme in biochar. Biochar 1, the biochar was immobilized with 0.1 g/L of enzyme; Biochar 2, the biochar immobilized with 0.25 g/L of enzyme; Biochar 3, the biochar immobilized with 0.5 g/L of enzyme.
| A enz (U/g) | Q p (g) | R (%) | |
|---|---|---|---|
| Biochar 1 | 0.1060 | 0.00251 | 23.6 |
| Biochar 2 | 0.1440 | 0.00700 | 44.4 |
| Biochar3 | 0.1926 | 0.01540 | 61.2 |
In order to determine the degree of enzyme affinity with the supported material the values of Vmax and K m were obtained. Using the equation 05, the K cat values for each support were calculated, and these results are shown in Table 2.
Table 2 Values of Vmax, K m and K cat for the biocatalysts.
| Vmaxa | Kmb | Kcatc | Kcat/Km | |
|---|---|---|---|---|
| Biochar 1 | 0.28 | 0.18 | 0.22 | 1.22 |
| Biochar 2 | 0.31 | 0.26 | 0.39 | 1.50 |
| Biochar 3 | 0.47 | 0.34 | 0.78 | 2.29 |
As already explained, the value of Vmax means the value where all active centers are saturated with substrate, there is no free enzyme to bind more substrate. It can be said that, for the enzyme concentrations studied, there is a good availability of active centers for the added enzymes, making them have a good enzyme-carrier bond.
According to Michaelis-Menten (1913), low values of the K m constant reflect on a high affinity of the enzyme for the substrate. Analyzing Table 2, it can be seen that for Biochar 3 support, a better result was obtained when compared to other materials. This is due to a greater amount of enzyme immobilized on the surface of the material. However, all the results showed a significant value, which shows, once again, the good affinity of the enzyme with the synthesized support 4), (23. The k cat value represents the number of substrate molecules converted into product per minute. The support Biochar 3 presented a much superior result and, consequently, more satisfactory with respect to the other materials. This support was able to convert about 0.78 g/min into fatty acids, followed by 0.39 g/min, converted by Biochar 2. It can be stated that a greater conversion of substrate into product is due to the greater amount of enzymes to do this task, that is, there is a greater amount of enzyme immobilized on the surface of the Biochar 3 support.
In order to determine the affinity between the enzyme lipase and the synthesized support, the specificity constant was calculated, K cat / K m . According to this constant, low values of the relationship between the catalytic efficiency of the enzyme, K cat , with its affinity for the substrate, K m , indicate little affinity of the enzyme for the substrate. As expected, the result obtained through this constant indicated that the lipase enzyme has a better efficiency when a greater amount of enzyme is used at the immobilization step.
Biochar from guava seeds, after chemical treatment with KOH, already presented as a good alternative to support the enzyme lipase 26. Two kinds of wood biochar were successfully used as a novel type of support for immobilizing laccase to promote the enzymatic degradation of HODiCB. The biochars prepared via a microwave assisted pyrolysis process possess proper porous structures. The immobilization improved the thermal stability of laccase and induced the enzymatic degradation of HO-DiCB effectively, thus leading to a reusable, cost-effective and green-based adsorbent 27. The functionalization of the biochar led to a surface modification of biochar by the insertion of amine-aldehyde groups, which was responsible for the formation of strong interactions between the support material and the pepsin enzyme, thus providing a greater proteolytic activity when compared to immobilization by adsorption. The immobilized enzymes were able to maintain their proteolytic activity for more than seven cycles of reuse 28.
The results and recent literature revealed the biochar have potential to became a novel support for the immobilization of enzymes, due the low cost in comparison with the established methods, making it a good alternative for applications on the industrial scale.
Conclusion
The objective of this work was to find out if the enzyme lipase has affinity with the biochar produced from cassava stump. It is known that many processes end up being expensive commercially due to the existing cost in the synthesis of the material used as support. When it is proposed to use an agricultural residue and to immobilize enzymes on its surface is something interesting not only from the economic point of view, but also environmental. This study showed that the lipase enzyme has an affinity with the studied biochar, presenting an immobilization yield in the 60% range when using 0.5 g/L of enzyme solution.