Introduction
The solitary bees Centridini Hymenoptera: Apidae are an endemic species in the Americas. This species has been reported as the most diverse group in the collection of oils, acting as pollinators in Malpighiaceae (Gaglianone, Rocha, Benevides, Junqueira, and Augusto, 2010). Most species nidify on the ground and usually in groups; however, some species as Centris tarsata (Smith, 1874), use holes in wood (Moure, Urban, and Melo, 2007). Some species builds its nests in these cavities, facilitating its collection through artificial nests. The collection method by artificial nests is easy to use, as it allows obtaining data on behavior and through the quantification of the number of species over time, it allows comparing and describing population changes of solitary bees (Aguiar and Garófalo, 2004; MacIvor, 2017).
Several studies on Centris seeking information about their ecology and behavior have been conducted by various authors (Magalhaes and Freitas, 2013; Martins, Peixoto, and Aguiar, 2014; Carvalho, Carreira, Rego and Albuquerque, 2016). Nevertheless, studies on the morphometry of these bees are still scarce (Ferreira, Aguiar, Costa and Silva, 2011). Among the various techniques used for studies on population, the morphogenetic and distribution of insects and, the geometric morphometrics has excelled as useful, accurate and low-cost tool, as reported in several studies (Ferreira, et al. 2011; Nunes, Araújo, Marchini and Moreti, 2012; Sousa, Araújo, Gramacho and Nunes, 2016).
The morphometric analysis is the statistical study on the covariance between shape changes and casual factors and differences in shape between organisms, whether phylogenetic or ecological (Monteiro and Reis, 1999). Geometric morphometrics methods offer graphical and analytical tools relevant to the quantification and visualization of morphological variation within and among samples of organisms (Alibert, Moureau, Dommergues, and David, 2001; Sigirli and Ercan, 2013). In bees, the wings are used in morphometric analysis for being flat structures and for presenting easy measurements of size and shape (Nunes, Pinto, Carneiro, Pereira, and Waldschmidt, 2007). Morphometry can be used to check sexual dimorphism between males and females based on body dimensions (Belleza and Demayo, 2014; Camargo, Camargo, Correa, Camargo and Diniz, 2015). Sexual dimorphism as well as evolutionary and ecological significance in insects has been investigated for many decades (Benitez, Briones and Jerez, 2011; Benitez and Vargas, 2017).
Factors linked to biology and behavior of insects, like dispersion capacity, can be elucidated through studies on evolution of wing sexual dimorphism. Wing geometry in flight capacity and dispersal of insects is of great importance as the wing morphometric pattern may be linked to changes in aerodynamics at the time of flight (Devicari, Lopes and Suesdek, 2011).
Therefore, the ecological-evolutionary explanation for the differences in shapes between males and females of C. tarsata is that females have a wing shape that can encourage their dispersion in search of floral features to their offspring and thus they fly greater distances. Males, on the other hand, need wings with a streamlined format that provides greater agility to achieve the female, allowing greater success in mating. Some authors also attribute the increased size of females to their ability to forage and fly faster and visit as many flowers per unit time, while related female size with foraging resistance in environments with environmental conditions (Belleza and Demayo, 2014; Sousa et al., 2016).
Camargo, et al. (2015) emphasizes that characters that differ between genders may show the occurrence of sexual selection, requiring further studies. Therefore, to explain this theory, it is important to deepen the research on the relationship between shape, size, function of the wings, as well as patterns that involve genetic inheritance of wing shape (Devicari et al., 2011). Studies on sex differentiation have great relevance in sex ratio, reproductive habits, as well as in the presence of ecological-evolutionary distinct factors between sexes. Therefore, this study aimed to analyze the morphometric variability and sexual dimorphism in Centris tarsata, collected from artificial nests installed in four different vegetation types.
Materials and methods
The monthly collections were held using trap nests made of Kraft paper tubes diameters of 7, 9 and 11 mm, with one end closed, grouped, and inserted into Styrofoam blocks. These blocks were fixed in wood pickets 1.5 m height and randomly installed in around areas: 1-Active Germplasm Bank AGB of Malpighia emarginata, 2-Other orchards Mangifera indica L., Musa spp., Spondias sp. and Citrus sp., 3-Forest fragment and 4-Intermediary transition area with Brachiaria sp. in the experimental field of Embrapa Cassava and Tropical Fruits located in the municipality of Cruz das Almas, Bahia State, Brazil 12°40’12” S, 39°06’07” W, 220 m.
Nidified blocks were identified and transferred to the Insect Study Group Insecta of Center for Agrarian, Environmental and Biological Sciences, Federal University of the Recóncavo of Bahia, kept in PVC pipe 20 cm, sealed with nylon screens to allow good aeration and packed in Biological Oxygen Demand BOD at 25 ± 1°C, humidity 75% ± 1% to monitor until emergence. The taxonomic identification was performad by PhD Favízia Freitas de Oliveira of the Federal University of Bahia. The right forewings of C. tarsata specimens that emerged in artificial nests were removed, arranged between two blades for microscopy, and photographed using a digital camera attached to a stereomicroscope with an increase of 7.5x using the software Motic 2.0 ML. The images obtained were processed in TpsUtil and then brought to the TpsDig2 version 2.12 for measurements from landmarks at the vein intersections according to Figure 1.

Figure 1 Forewing and landmarks located at wing vein intersections used by morphometric analysis in Centns tarsata Hymenoptera: Apidae.
Subsequently, the data were generated, extracted, and transferred to software MorphoJ to perform the statistical analysis. The Canonical Variables CV compared the populations of females and males and the Principal Component Analysis PCA checked sexual dimorphism. In order to confirm the accuracy of the data generated from the CV, as well as the correct classification of each individual within each group, cross-validation was performed. The results of the analyses were obtained by means of the software PAST and R, respectively. In addition, the ANOVA test was conducted to analyze centroid size and verify the significance of the results. A Tukey test was chosen to compare the means of distinct populations.
Results
The canonical variables explained 51.81% for the first variable, 36.54% for second, and 11.65% for the third, totaling 100% of data variation in males. For females, the first variable explained 44.04%, the second 36.74% and the third 19.20%, totaling 100% variation of the data for the area studied. This result confirms an effect of the environment on the intrassexual morphometric pattern (Figures 2 and 3).

Figure 2 Analysis of canonical variables of Centris tarsata males in different geographic areas: 1-Active Germplasm Bank AGB, 2-Other orchards, 3-Forest fragment and 4-Intermediary.

Figure 3 Analysis of canonical variables of Centris tarsata females in different geographic areas: 1-Active Germplasm Bank AGB, 2-Other orchards, 3-Forest fragment and 4-Intermediary.
The cross-validation test ranked correctly 57% of total male groups, highlighting that the highest rating percentage was observed in acerola orchard reaching 70.83%. For female, the test correctly classified 62% of the total, and individuals that more differentiated were found in the forest fragment the equivalent to 74%. These results suggest that some resources used by C. tarsata in the acerola orchard and in the forest fragment influenced intrassexual differentiation in bees from this species, when compared to the bees collected in other locations.
According to the Procrustes distance for wing of C. tarsata between the areas, males there were different for the localities Inter x Other Orchards and Forest x Other Orchards P < 0.05; and between Inter x acerola, Other orchards x acerola P < 0.01 (Table 1). For females, there was different between all groups (P < 0.01 Table 2). According to the Mahalanobis distance for the size of individuals, there were significant difference (P < 0.01) in males and females between study areas Tables 3 and 4. The analysis of variance was significant P < 0.05 for the centroid size and it showed that individuals of C. tarsata had variations in size depending on the location of nesting and sexual dimorphism, where females presented larger wings than males did Figure 4. In the PCA, the first component was 36.77%, second 9.82%, and third 8.08% to sexual dimorphism, of the total data variability was 54.67% (Figure 5).

Figure 4 Centroid size of wings of Centris tarsata by gender and areas: 1-AGB of M emarginata, 2-other orchards, 3-Forest fragment and 4-Intermediary. Same letters indicate that there is not difference between females and males groups into the different areas, by the Tukey test at 5% probability.

Figure 5 Males and females dispersion of Centris tarsata according to Cartesian axes established by the first and second principal components. And Thin-Plate spline representing morphological extremes in the first principal component PCA1. The vectors indicate the direction of the variation of each landmark.
Table 1 Procrustes distance lower and statistical significance P by distance top for males between áreas through geometric morphometrics*.

* AGB of acerola Malpighia emarginata, other orchards Mangifera indica, Musa spp., Citrus sp., Spondias sp., forest and inter = intermediary transition area with Brachiaria sp.
Table 2 Procrustes distance lower and statistical significance p by distance top for females between areas through geometric morphometrics*.

* AGB of acerola Malpighia emarginata, other orchards Mangifera indica, Musa spp., Citrus sp., Spondias sp., forest and inter = intermediary transition area with Brachiaria sp.
Table 3 Mahalanobis distance lower and statistical significance p by distance top for males between areas through geometric morphometrics *

* AGB of acerola Malpighia emarginata, other orchards Mangifera indica, Musa spp., Citrus sp., Spondias sp., forest and inter = intermediary transition area with Brachiaria sp.
Table 4 Mahalanobis distance lower and statistical significance p by distance top for females between areas through geometric morphometrics *
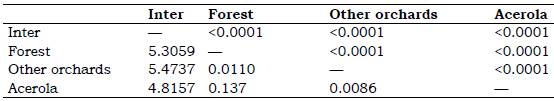
* AGB of acerola Malpighia emarginata, other orchards Mangifera indica, Musa spp., Citrus sp., Spondias sp., forest and inter = intermediary transition area with Brachiaria sp.
Discussion
Neves, Carvalho, Souza and Lima, 2012 observed significant differences between individuals of Tetrapedia diversipes Klug Hymenoptera: Apidae per area and suggest that the use of various resources can influence the morphometric pattern among bees. Studies by Buschini and Wolff (2006) and Carvalho, et al., (2016) showed that females of C. tarsata were larger than males, using head width measurement. Sousa et al. (2016) noted that environmental conditions, such as seasonal variations, trophic resources and nesting, may favor the occurrence of sexual dimorphism related to size. Camargo, et al., (2015) suggest that sexual dimorphism correspond a feeding adaptive strategy adopted by the mother, where the female may allocate the cells with greater amounts of food to the gender with a larger size. Roulston and Cane (2000) observed that males in the larval stage consume less food, providing a smaller size due to the confined space of their cells in relation to female cells. Moreover, sexual dimorphism linked to body size is regarded as a main factors related for reproductive success (Belleza and Demayo, 2014).
Males collected in the forest fragment showed variation in wing size. In the forest fragment, trophic availability and preferred resources of C. tarsata is smaller than other areas studied (Figure 4), which explains the rank of males in this area based on cross-validation, as mentioned above. However, keeping forest edges around orchards of plants that provide resources for the Centris, like acerola, is essential to offer other features such as availability of natural cavities for nesting, favoring the maintenance of the population these bees in agroecosystems. Although this variation in wing size was not observed for females in forest fragment, it’s possible that scarcity of trophic resources, female feeding is favored at the expense of male feeding, since there is a trend in which the females spend and need more energy during their active life than males do and thus requiring a greater quantity of food. Roulston and Cane (2000) observed that variations in body size are linked to the quantity and food quality of larvae and transmitted over generations, suffering greater influence of environmental factors. Also, females could control the sex of their offspring according to the availability of resources.
In bees, body size affects the features associated with the individual adaptive value. A larger body size for females could be linked to reproductive success, providing greater capacity to feed the cells, lay eggs, and ensure the offspring. In males, the larger size could be linked to success in the displacement of competitors, increasing reproduction chances (Neves, et al., 2012). Andersson (1994) points out that through the intrasexual selection, larger males tend to be stronger, more competitive, and with higher reproductive success.
Differences between wing shapes of males and females confirm with results showed by Devicari, et al., (2011). The authors found that the phenotypic expression of the wing shape was specific to males and females of C. tarsata and can be independent of sampling site, where there was a grouping between individuals of the same sex. In other studies, geometric morphometrics was also effective to detect sexual dimorphism in T. diversipesNeves et al., 2012 and Aedes scapularis Diptera: Culicidae (Devicari, et al., 2011).
Neves et al. (2012) emphasize that there are ways to detect sexual dimorphism as through secondary sexual characteristics differences in morphology, morphometry, and behavior. However, most studies on dimorphism emphasize the differences in body dimensions (Andersson, 1994). The wing shape has greater evolutionary stability compared to size (Nunes, et al., 2012). Wing has high heritability with minimal changes by non-genetic factors, unlike the size factor of other body structures that can to vary by plasticity and environmental conditions such as food quantity, temperature and humidity.
Conclusions
The morphometric intrasexual for individuals of Centris tarsata was influenced by environmental conditions and promote sexual selection.
Centris tarsata showed sexual dimorphism for wing size and shape, which can promote and determine distinct biological characteristics in an evolutionary process.
These data could contribute to a better understanding of the evolutionary process, as well as of ecological, biological, morphogenetic, and behavioral factors associated with the role of the bee in the environment.














