INTRODUCTION
The production of Theobroma cacao in Ecuador, and elsewhere in the America, is affected by the diseases of Frosty Pod Rot (Moniliophthora roreri) and Witches' Broom (Moniliophthora perniciosa). These pathogens are highly aggressive once they come to infect the crop. In the case of M. roreri, mainly affects the fruits in any state of development, which cause severe yield losses in the production (30-100 %) (Bowers et al., 2001; Sánchez et al., 2015). Similarly, M. perniciosa causes considerable losses of production in Ecuador (60 %-70 %) (Meinhardt et al., 2008) as it affects leaves, branches, shoots, flowers and young developing fruits (Parra and Sánchez, 2005).
At present, for the control of diseases in agricultural crops there are alternatives such as biological control, which consists of the use of antagonistic microorganisms against specific diseases and pathogens (Bailey et al., 2008; Jaimes and Aranzazu, 2010). In this research, we try to find endophytic fungi associated with the same crop for the control of the two major diseases in T. cacao. Endophytic fungi are of great importance because they have the ability to produce bioactive metabolites that the plant uses as a defense mechanism against attack by pathogen (Arnold et al., 2003). Studies with native microorganisms as antagonists, both bacteria and fungi, have been shown to be effective for the control of M. roreri and M. perniciosa (Hebbar, 2007; Bailey et al., 2008; Suárez and Cabrales, 2008; Krauss et al., 2010; Hernández-Rodríguez et al., 2014).
In Ecuador, several trials have determined that integrated management is the best tactic for the control of frosty pod disease and the witch's broom (Solis and Suárez-Capello, 2006; Saquicela-Rojas, 2010). These trials not only include the use ofbiological products (Trichoderma spp.) or chemicals (chlorothalonil, cupric oxide, azoxystrobin or copper sulphate), but pruning and removal of diseased fruits. The commercial availability of strains with demonstrated biological activity are limited to the areas of influence of some institutions, or when commercial products are available, there are no additional guarantee elements of the effectiveness of the strains in the field beyond the registration requirements of the product (MAGAP, 2016). In this framework, concerning the management of diseases in various crops, and especially cocoa, the CIBE has developed the production of a biol in a standardized manner for several years (Chávez and Peralta, 2016) in order to recover old cocoa plantations, through the enhance the vigor of plants and to improve the phytosanitary state of plantations. At the same time, it seeks to develop a biological control agent based on endophytic fungi species. The present investigation pursues this last objective. The aims of the study were to determine for 17 endophyte strains: i) the mycoparasitism against M. roreri and M. perniciosa, ii) the production of inhibitory metabolites to Moniliophthora spp. and iii) the ability to recolonize healthy leaf tissue of the host through laboratory test.
MATERIALS AND METHODS
Origin of endophytic and pathogenic strains
The 17 strains for this study were isolated from healthy leaves of variety of Nacional type cacao with more than 50 old years. Two localities from Guayas province: Balao (2°30'29.5''S, 79°46'34.8''W) and Naranjal (2°40'35.2''S, 79°38'21.2''W) and one from Azuay province: Molleturo (2°30'49.2''S, 79°26'11.2''W) were sampled. The endophyte strains showed higher percentage of growth inhibition (PGI) against M. roreri and M. perniciosa and it was also found that they were not pathogenic of fruits and leaves of T. cacao (Villavicencio-Vásquez, 2018). Strains of M. perniciosa (CIBE-MP22) were isolated from Vinces and M. roreri (CIBE- A12.1) from the Amazon region (Table 1).
Mycoparasitism Test
Dual cultures of endophytes-pathogens in plates containing potato dextrose agar (PDA) (4 g potato, 20 g dextrose and 15 g agar) medium, at pH 6.2 incubated at 26° C in darkness were performed (Condori et al., 2016). Two replicate plates were prepared for each strain of endophyte fungi. After 13 days of incubation, ten discs (5 mm) from the interaction zone of each two reply were extracted and plated in another PDA-plate, incubated as described before for seven days, and observed for the growth of the endophyte or the pathogen (Fig. 1). Mycoparasitism of each strain was determined based on the percentage of success in the survival (growth) of the pathogen with respect to the total of discs of the two replicates (% mycoparasitism = N° discs with pathogen growth /20 discs x 100).
Soluble inhibitory metabolite production
The fungal metabolites were obtained in liquid Czapeck medium, containing 2 g NaNO3, 5 g KH2PO4, 0.5 g MgSO4, 10 mg FeSO4, 3 mg ZnSO4, 1g Yeast Extract and 60 g Glucose per liter at pH 7. Three flask containing 150 ml of medium were each inoculated with four mycelial discs of the endophyte of a 7-day-old colony in PDA. Three replicates were used for each treatment (strain). The cultures were incubated at laboratory room temperature (« 25 ± 2 °C) in an orbital shaker (110 rpm/21 d). After 21 days growth, mycelia were collected and the liquid sterilized in a vacuum system through a 0.22 jm filter (Millipore). The test was carried out with two concentrations (50 % and 75 %) of crude extract fungus diluted with PDA medium and poured into Petri dishes and plates containing media mixed with sterile distilled water were included as control. Once the filtrate solidified, agar discs (5 mm) of the pathogens (7-day-old) were placed at the center of the Petri dish. Three replicate plates of each concentration for each endophyte metabolite were incubated at 26 ° C in the darkness. The percentage of inhibition of mycelial growth of the pathogens were recorded as the difference between the average radial growth in the presence and absence of fungal filtration using the formula proposed by (Ezziyyani et al., 2004).

Figure 1 Examples of dual cultures of M. roreri vs. endophytes after eight days of incubation (A1-C1). A1, vs. strain Ec107 (C. gloeosporioides; B1, vs. strain Ec129 (N. sphaerica) and C1, pathogen alone. Re-isolation (survival) of the “winner” fungi from the agar-plugs extracted throughout the colony-contact areas (A2-C2). Series 2 shows plates with 0, 50 and 100 % of survival of M. roreri after the confrontations, respectively.
PGI = ((R 1 -R 2) / R 1) * 100 [Formula 1]
Where: R1 is the radius of control pathogen and R2 is the pathogen with diluted crude extracts.
Ability to recolonize healthy leaf tissue of T. cacao
The ability of each strain to colonize healthy cacao tissue was studied. Seven days old cultures in Petri dishes of each of the 17 strains were photographed. Next, each plate was scraped and 5 mL of 0.05 % Tween 20 was added to aid mycelium transference. The mycelium was macerated, and the concentration adjusted to 1 06-107 CFU ml-1 with sterile distilled water. For this test, detached leaves from seedlings of varieties of Nacional type cacao were obtained from the Instituto Nacional de Investigaciones Agropecuarias, INIAP (2°15'15''S, 79°49'W) in Guayas, Ecuador. Detached leaves were chosen to be as similar as possible in terms of size and age (two months old). Then were disinfected with 2 % sodium hypochlorite for two minutes and washed with sterile distilled water (Bañuelos-Balandrán and Mayek-Pérez, 2008). The leaves were uniformly sprayed with a volume of 1.5 mL of the inoculum solution. The leaves sprayed with sterile distilled water served as control. Each leaf was incubated in a plastic bag containing wet paper towels to maintain 100 % relative humidity (Tahi et al., 2007). The leaves were incubated for seven days in dark at 26 °C at an incubation chamber. After incubation, the inoculated leaves were washed with tap water, then three discs of 5 mm from each side of the central vein were extracted and disinfected as described above. Each leaf disc was cultured in Petri dishes containing 2 % malt extract agar (12.75 g maltose, 2.75 g dextrin, 2.35 g glycerol, 0.78 g peptone and 15 g agar) according to the methodology described previously (Arnold and Herre, 2003). After the third day of incubation, all mycelium that emerged from each leaf disc were transferred to a new PDA-plate. Three leaves and 18 leaf-discs for each treatment were used.
The identification of the colonies was based on three macroscopic characteristic: color, shape and texture of the colonies, before inoculating and after the posterior reisolations. Digital photographs were taken at each stage. The percentage of colonization of each endophyte was determined (% =N° discs with the re-isolated-endophyte-strain/18 total discs seeded * 100)
Data analysis
The mycoparasitism, PGIs obtained in the crude extracts and the re-isolation of the endophyte in the detached leaves, were compared using a non-parametric analysis of variance (Kruskal-Wallis) with a level of significance of 0.01, since after several transformations they did not present normal distribution using Shapiro-Wilks test. All analyses were carried out using the statistical package InfoStat (Di Rienzo et al., 2008).
RESULTS
Micoparasitism
The figure 2 shows the percentage of survival (growth) of M. roreri and M. perniciosa after the confrontation with the endophytes. Ten endophytes strains prevented the growth of both pathogens (Ec027, Ec035, Ec067, Ec084, Ec098, Ec107, Ec113, Ec120, Ec151 and Ec157). The other seven endophytes showed variable response to the pathogens (Fig. 2).
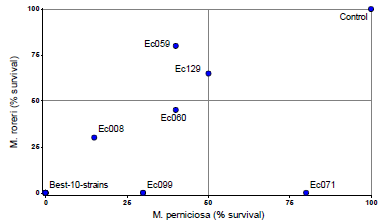
Figure 2 Percentage of survival of M. roreri and M. perniciosa after 13 days of confrontation with endophytic strains in dual-culture-assays. The values were determined based on the number of disc that showed growth of the pathogens when extracted from the interaction zone of the endophyte-pathogen colonies. The lower %-value, the more mycoparasitic capacity of the endophytes. The 10 best isolates are represented by a point with 0 %-0 %. Colonies were cultivated on PDA (26° C/darkness), and two replicates and 20 discs per treatment were used.
Soluble inhibitory metabolite production
The crude metabolites of 11 and seven out of the 17 strains were able to inhibit the growth of M. roreri and M. perniciosa at both concentration, respectively (Fig. 4). The strain Ec107 at the 75 % (v/v) of crude extracts showed the greatest inhibition (60 %) against M. roreri (Figs. 3A and 4A), whereas the same extracts had the opposite effect on M. perniciosa, that is, it stimulates the growth of the pathogen, showing the negative values (Fig. 4B). The second more inhibitory strain (Ec035) against M. roreri at the same concentration, showed the highest inhibition against M. perniciosa (47 %) (Fig. 3C and Figs 4A-4B). In the figures 3B and 3D, the controls are shown. On the other hand, crude metabolites extracts of six strains always stimulate the growth of the pathogens at both concentrations, Ec084 (Daldinia eschscholtsii) against M. roreri, and five other including Ec084 against M. perniciosa (Fig. 4A-4B). Seven out of ten strains with the highest mycoparasitic capacity of the mycelium against both pathogens, also inhibited the growth of both pathogens through their crude metabolites (Ec027, Ec035, Ec067, Ec098, Ec151 and Ec157).

Figure 3 Growth of M. roreri onto PDA medium combined with 75 % crude metabolites of: A, strain Ec107 (C. gloeosporioides); C, strain Ec035 (L. theobromae). B and D, Controls with no extract of M. roreri and M. perniciosa, respectively.

Figure 4 Effect of endophytic fungi metabolites on the micelial growth: A. M. roreri and B. M. perniciosa. Crude extracts of the metabolites from liquid Czapeck medium at 25° C ± 2 for 21 days. Negative values indicate stimulation of the pathogen respects to the control (PDA without the metabolites). Different letters indicate significant differences (p <0.01).
Ability to recolonize healthy leaf tissue of T. cacao
Nine out of the 17 endophytes were not recovered from the detached leaves (Fig. 5). Three strains achieve the infections of the leaves and were recovered from 100 % of healthy-leaf-discs: Ec059 (X. feejeensis), Ec098 (L. theobromae) and Ec151 (L. theobromae), showing the highest host colonizing capacity. Six out of seven strains whose metabolites inhibited the pathogens at both concentrations, and showed grater mycoparasitism by confrontation of the colonies, were recovered from the leaf discs of the host (Fig. 5). These strains were Ec098, Ec151, Ec157, Ec120, Ec067 and Ec027, however, as best strains should be considered the first three with re-isolation percentages between 80 and 100.

Figure 5 Percentage of the re-isolation of endophytes from discs of inoculated detached healthy-cacao-leaves. Detached leaves were incubated for seven days at 26° C (darkness, 100 % RH). Average from 18 leaf-discs from each strain are showed. Different letters indicate significant differences (p <0.01).
In the present study, the strains with the best simultaneous results in the three variables analyzed were Ec098, Ec151 and Ec157, all belonging to L. theobromae. These strains reinfected the host tissue between 80 %-100 % of the times, and maintained inhibitory effects both at the level of interaction of the colonies (mycoparasitism) and by their metabolites against M. roreri and M. perniciosa. On the other hand, the strain Ec035 (L. theobromae) stood out for its inhibitory effects in the trials of mycoparasitism and metabolites against both pathogens; and Ec107 (C. gloeosporioides) for inhibiting in 60 % the growth of M. roreri with 75 % (v/v) metabolites. However, at the same time, Ec107 metabolites stimulate the growth of M. perniciosa (Fig. 4). Neither of the two last strains could be recovered from the host.
DISCUSSION
More than 50 % of the strains could not be recovered from the host tissue. Of the eight strains that reinfected the leaf tissue, six belonged to L. theobromae (Ec098, Ec151, Ec157, Ec067, Ec120 and Ec027) and two to Xylaria spp. (Ec059 and Ec099). Both taxa have been reported as frequent colonizers in natural ecosystems (Arnold et al., 2003). It was noted in our experiments that C. gloeosporioides s.l. does not recolonized the host tissue, when studies carried out on natural ecosystems in Panama have shown it as an abundant endophytic species (Arnold et al., 2003).
Mejía et al. (2008) reported that C. gloeosporioides s.l. had high colonization capacity in leaves of cacao seedlings in greenhouse and field trials. The endophytes possesses characteristics of colonizing ability that generate benefits to the host plant according to the different mechanisms that it possesses, such as: antagonistic activity, induction of resistance against pathogens and promotion of growth through the secretion of phytohormones (Harman et al., 2004).
Fungal metabolites of endophytes can control the growth of pathogens through mechanisms involving chitinolytic and glucanolytic enzymes that hydrolyze the components of pathogen's cell walls (Harman, 2006) and also may contain antibiotics or toxins that enhance biocontrol efficacy against particular host-pathogen interactions (Howell, 2003). Within L. theobromae, secondary metabolites (jasmonic acid) seems to play a key role against the fungus Sclerotium rolfsii (Michelena et al., 2005) and within C. gloeosporioides, colletotric acid it is responsible for antifungal activity against Helminthosporium sativum (Zou et al., 2000).
A combination of endophyte fungi can reduce the damage caused by Moniliophthora spp. (Arnold et al., 2003). Ideally, we should search for endophytes that combine diverse mechanisms of antagonistic action, such as a good growth rate, some degree of antibiosis and good colonization and survival in the tissues of the plant (Mejía et al., 2008; Hernández-Rodríguez et al., 2014). Studies combining six endophytic species associated with cacao plants reduced the damage caused by Phytophthora sp. (Arnold et al., 2003). Other studies reported combination of strains of Xylaria spp. effectively limits damage caused by pathogens in T. cacao (ZhiLin et al., 2009).
CONCLUSIONS
It should be convenient to combine several strains in a biological control agent formula, both for the advantages that can be given when applying a biological control agen in different agroecosystems, and for the facts observed in this report that not all strains were optimal in their performance. The strains that were consistent in the three variables analyzed and against both pathogens should be verified in planta experiments. The other two strains, Ec035 and Ec107, should be investigated to find specific metabolite active against MR or MP pathogens.















