Introduction
Movement disorders can occur after a cerebrovascular event, either in an early or late. Up to 22% of secondary movement disorders are attributed to cerebrovascular disease. Both ischemic and hemorrhagic cerebrovascular events can lead to post-stroke movement disorders (PSMD), but a higher incidence has been reported in ischemic lesions 1-2.
The phenomenology of the movement disorder varies based on their location and the etiology. Hemiballismus may present as an initial or preceding symptom of cerebrovascular disease 1,3. The location of lesions that can precipitate PSMD can vary depending on the age group of the affected individuals. The exact pathophysiology underlying the differential development of PSMD in patients with similar radiological and anatomical features remains unknown 4.
We present the case of a patient who developed sudden-onset hemiballismus with an atypical clinical course, accompanied by documented thalamic injury. This is followed by a review of relevant literature.
Case presentation
An 82-year-old male with no formal education, accompanied by his daughter, presented to the emergency department reporting "generalized weakness". The patient and his daughter described an initial episode of weakness that significantly impaired his ability to walk, which resolved spontaneously and completely. Approximately three hours later, he experienced a second episode characterized by sudden-onset involuntary movements on the left side of his body. These movements persisted during assessment by the neurology department.
The patient had no documented history of neurological complaints or movement disorders and had previously maintained independence and functional capability in both basic and instrumental daily activities. His past medical history included chronic obstructive pulmonary disease requiring supplementary oxygen, hypertension, and insomnia. He was on antihypertensive medications and used inhalers for his lung disease. The general physical examination yielded no relevant findings.
The neurological examination revealed mild cognitive impairments, dysarthria, a left extensor plantar response, and irregular, sudden, unpredictable involuntary movements. These movements displayed a wide range with a proximal predominance on the left side of the body, including the facial structures. Given the abrupt onset of the clinical presentation and the patient's cardiovascular risk profile, the primary concern was to rule out an acute neurovascular syndrome. The National Institute of Health Stroke Scale (NIHSS) score was 0 points (the dysarthria was attributed to hyperkinetic involvement of the facial muscles).
A plain head computed tomography (CT) scan revealed changes consistent with leukoencephalopathy (Figure 1). The scan showed parenchymal hypodensities corresponding to chronically evolved infarcts in the lenticular and thalamic-capsular regions on the left side, as well as a right thalamic hypodensity indicating a subacute vascular injury. Symptomatic management with a low dose of risperidone was initiated. While the involuntary movements persisted, their frequency diminished, and they were regarded as less bothersome by the patient.
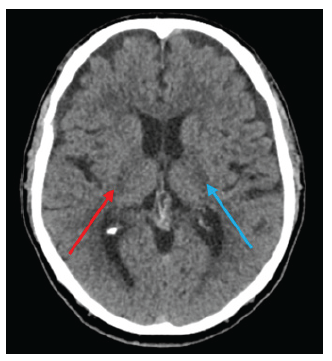
Note: The red arrow indicates the subacute lesion on the right, while the blue arrow points to the chronic lesions identified by the physician team.
Source: Patient's medical record, modified and shown with previous consent.
Figure 1 Plain head CT scan at the time of presentation
During the patient's hospitalization, a cardiovascular risk assessment revealed a nonreactive non-treponemal (VDRL) test and an altered lipid profile with elevated LDL and cholesterol levels, while HbA1c levels were normal. A carotid ultrasound showed calcified atheromatous carotid disease, and an echocardiogram indicated no ventricular dysfunction, with preserved left ventricular ejection fraction and no atrial dilation.
Seventy-two hours after the initial consultation, the patient exhibited neurological deterioration, including hemianopia, facial droop, worsening dysarthria, dysphagia, left hemiparesis, and left hypoesthesia, resulting in an updated NIHSS score of 12. A follow-up head CT revealed a slight increase in the right thalamic hypodensity (Figure 2).

Note: There is a notable increase in the low-density areas along the posterior capsular region, along with cytotoxic edema in the anterolateral thalamic region.
Source: Patient's medical record, modified and shown with previous consent.
Figure 2 Control plain head CT taken after deterioration in the NIHSS score
The patient underwent a brain magnetic resonance imaging (MRI) scan, which revealed genuine diffusion restriction in the lateral thalamus, lenticular nucleus, and right internal capsule (Figures 3 and 4). Additionally, white matter disease was identified and classified as Fazekas I.
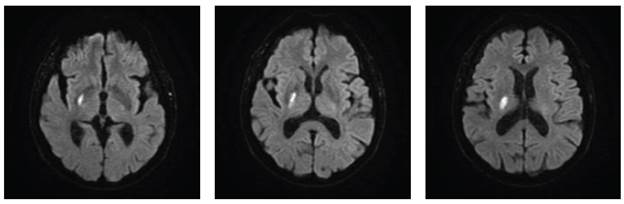
Note: Diffusion restriction is observed in the right lateral thalamus, as well as in the lenticular and posterior capsular regions on the right side.
Source: Patient's medical record, modified and shown with previous consent.
Figure 3 Simple brain MRI in axial view, DWI sequence

Note: Diffusion restriction is observed in the right lateral thalamus, as well as in the lenticular and posterior capsular regions on the right side (indicated by red arrows). These findings corroborate those seen on the DWI sequence.
Source: Patient's medical record, modified and shown with previous consent.
Figure 4 Simple brain MRI in axial view, ADC sequence
After completing the cardiovascular risk assessment, the event was attributed to small vessel disease.
The patient was discharged with scheduled follow-up appointments with neurology, physical medicine, and internal medicine. Risperidone was discontinued as it was no longer beneficial. Prescriptions for multimodal rehabilitation therapy were provided, and the patient also received pharmacological secondary prevention.
Literature review and discussion
Globally, post-stroke movement disorders (PSMD) are observed in 1 to 4 percent of all cerebrovascular diseases, with equal prevalence between males and females. Among movement disorders associated with cerebrovascular disease, chorea (with or without accompanying ballismus) and dystonia are the most frequent 1-2.
Sudden-onset chorea, such as that presented by our patient, can be caused by infections, autoimmune diseases, neurodegenerative diseases, and stroke, with stroke being the most frequent cause of sporadic chorea. It can also present as a pre-stroke symptom and has been reported in the absence of infarction, in association with middle cerebral artery stenosis 3.
The incidence of stroke-related choreiform movements varies in the literature. Chorea and ballismus have been reported as the second most frequent type of PSMD in a systematic review of literature, representing 16% of the overall phenomenology reported 1. In other studies, the incidence has been as low as 0.54% 5. Age has been identified as a predisposing factor, with a higher incidence observed in patients over 70 years of age 1,6.
The latency between the onset of stroke and the development of movement disorders varies, ranging from one day to several years after the event 7. Chorea is reported as the fastest-onset PSMD, often appearing within 24 hours of the stroke 1.
Lesions at a subcortical level, specifically in the basal ganglia and thalamus, are most commonly associated with the development of hemidystonia, hemichorea, and hemiballismus 7. Chorea and ballismus have been linked to lesions in the posterior and paramedian thalamus. A case-control study found that lesions affecting the caudate nucleus were statistically associated with post-stroke hemiballismus 6. Imaging studies have established associations between lacunar infarcts in the putamen and the caudate nucleus contralateral to the symptomatic side and the development of hemichorea/hemiballismus. Additionally, these symptoms have been reported in relation to subthalamic nucleus (STN) lesions 4. Pure cortical strokes have also been identified as a cause of hemiballismus 5.
The pathogenesis of post-stroke hemiballismus has not been fully elucidated. One hypothesis suggests that compromise of the STN can lead to under-activation of the internal globus pallidus (GPi) and subsequent thalamic disinhibition, resulting in excessive movement of the contralateral side. However, this hypothesis has been questioned due to the topographical variability and the fact that not all patients with STN lesions develop hemiballismus in real-life case series 6. Other researchers propose a network-based pathogenesis. Focal damage from stroke can disrupt one or more neuronal networks, leading to a spectrum of motor deficit 6. For instance, disruption of the sensorimotor pathway -particularly the network connecting the parietal lobe and the insula- can impair sensorimotor integration and result in post-stroke hemiballismus 8.
The clinical heterogeneity observed in patients with similar topographical lesions -some with hemiballismus and some without- has led to the hypothesis that individual susceptibility, including neuronal plasticity within the basal ganglia and compensatory mechanisms, may protect against the loss of motor control 7,9.
Regarding the prognosis of PSMD, more than 70% of patients show improvement or complete resolution of symptoms. Among those who continue to experience movement disorders, about 50% benefit from symptomatic treatment 1. Antidopaminergic medications, such as haloperidol or risperidone, have been reported as effective therapeutic strategies 6, as have GABA agonists. However, their use should be limited to cases that are persistent, functionally disabling, and bothersome. The MDS recommends an expectant approach, given the tendency for symptoms to resolve within weeks or months 9. In some cases, movement disorders may present progressively, with patients initially displaying hemiballismus that evolves into hemichorea and eventually into hemidystonia. The overall prognosis depends on the type of abnormal movement, as well as other factors such as age, comorbidities, and access and response to pharmacological therapies 7.
In our research, we found several reports of hemiballismus of vascular origin that resolved spontaneously. However, none of these cases showed clinical deterioration after imaging confirmation, unlike the situation observed in our patient.















