Introduction
Primary anal canal cancer is a rare condition, accounting for 1% to 3% of all gastrointestinal cancers1,2. The most common histological type is squamous cell carcinoma, with an incidence of approximately 0.5 to 2 cases per 100,000 inhabitants. The primary risk factor is infection with the human papillomavirus (HPV), which is responsible for 85% to 93% of all cases, particularly subtypes HPV 16 and 182.
Symptoms are nonspecific, including rectal bleeding, pain, and itching, which are associated with a delayed diagnosis. Digital rectal examination is an effective and low-cost method for detecting these lesions, and a definitive diagnosis should be made via biopsy. Studies, including pelvic magnetic resonance imaging with a protocol for the anus, allow for locoregional evaluation and staging, in addition to chest and abdominal CT scans to assess distant extension.
Neoplasms of the anal canal have a prognosis related to tumor staging. Localized stages can be treated with curative surgery; however, in locally advanced stages, the treatment consists of concurrent chemotherapy and radiotherapy with curative intent3. Tumors in advanced stages (IIIC and IV) have a poor prognosis, even with chemotherapy management.
In the last 10 years, both immunotherapy and targeted treatments with checkpoint inhibitors have generated new therapeutic perspectives, improving the life expectancy of patients in the context of advanced, recurrent, or refractory disease to conventional treatments.
Case Description
This case involves a female patient from Bogotá, Colombia, with a history of hypothyroidism on hormone replacement therapy, who, at the age of 69, presented to Fundación Santafé de Bogotá with a clinical picture of several months’ duration, characterized by anal pain and changes in the characteristics of her stools. She was evaluated in the emergency department, where a pelvic magnetic resonance imaging scan with an anal protocol was requested. The results reported irregular and transmural thickening of the anal canal walls on the left side, between the 12 o’clock and 6 o’clock positions, with involvement of the external anal sphincter extending to the dentate line and measuring 40 mm in length. Additionally, two prominent lymph nodes were identified in the left internal iliac chain, measuring approximately 11 x 17 mm in diameter, and in the left external iliac chain, measuring 14 x 11 mm (Figure 1), which showed a moderate pattern of diffusion restriction with suspicious characteristics (09/03/2019). Contrast-enhanced CT scans of the chest and abdomen were performed, revealing no abnormalities suggestive of metastatic disease, and a biopsy of the described lesion was conducted.
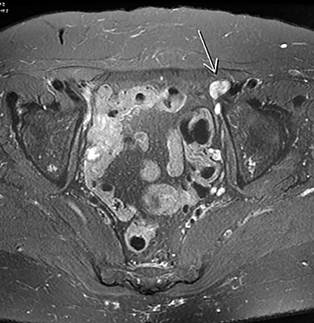
Source: Author’s File.
Figure 1 Initial magnetic resonance imaging showing lesions consistent with anal cancer in a locally advanced stage.
The diagnosis of moderately differentiated keratinizing squamous cell carcinoma of the anal canal with involvement of the distal rectum was confirmed; T2N1M0 (stage IIIA) by September 2019, with immunohistochemistry reporting a programmed cell death ligand 1 (PD-L1) combined positive score (CPS) of 10 points. The patient was referred to the oncology service, and definitive management for locally advanced disease was defined using the Nigro regimen, which consisted of intensity-modulated radiation therapy (IMRT) with a total dose of 50.4 Gy delivered in 1.8 Gy fractions, along with concurrent mitomycin and capecitabine during the radiation treatment days.
Three weeks of treatment were completed, but local toxicity to the radiation therapy led to suspension of treatment. A follow-up pelvic magnetic resonance imaging was performed four months after treatment cessation, which reported persistent irregular thickening of the anal canal walls on the left side, between the 12 o’clock and 6 o’clock positions, with involvement of the internal sphincter, intersphincteric space, external sphincter, and dentate line measuring approximately 35 mm (compared to 40 mm in the previous study), with involvement of the perianal fat and the posterior wall of the adjacent vagina. Compared to the prior study, these findings showed a moderate decrease in the vascularization pattern, with no evidence of lymph nodes or adenopathy in the left inguinal region.
She was presented in a multidisciplinary meeting with the coloproctology service, and it was determined that an abdominoperineal resection with colostomy would be performed. The procedure was carried out in March 2020, with a pathological report of the surgical specimen indicating ypT4, ypN1a, compatible with the absence of clinical response, leading to a decision for strict follow-up and observation.
In October 2020, a re-evaluation was conducted using a positron emission tomography-computed tomography (PET-CT), which revealed an increase in the density of the left paraaortic retroperitoneal fat, with a hypermetabolic image approximately 15 mm in size and a maximum standardized uptake value (SUV) of 7.32, related to surgical sutures, near the inferior mesenteric vessels, likely tumor-related, with no other pathological findings (Figure 2).
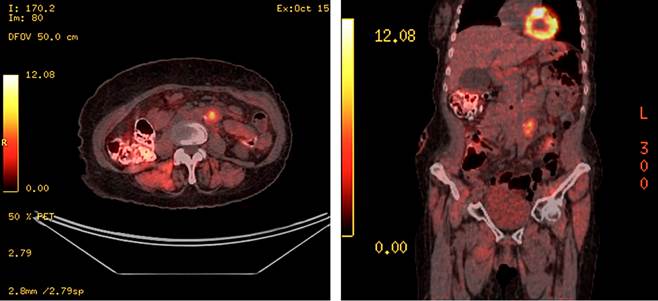
Source: Author’s File.
Figure 2 Follow-up positron emission tomography (PET) demonstrating disease progression.
Locoregional progression was considered, and treatment was initiated with systemic chemotherapy, adjusting the doses of paclitaxel and carboplatin to 85% (80 mg/m²). Treatment began in October 2020, and after three cycles of chemotherapy, a pelvic magnetic resonance imaging was performed in December 2020, showing evidence of a solid nodular lesion in the left paraaortic retroperitoneum measuring approximately 15 x 21 mm, with a metastatic neoplastic appearance, located at the level of the distal bifurcation of the abdominal aorta and at the level of the L4 vertebral body. Compared to the previous CT study, this lesion exerted greater compressive effect on the ipsilateral ureter, causing proximal renal obstruction with mild to moderate hydronephrosis.
This was considered a progression of the disease, and a second-line treatment with capecitabine and oxaliplatin was proposed, which began in December 2020. After administering the third cycle, she was presented in a multidisciplinary meeting, where it was determined that, based on the initial pathological report with a CPS of 10, she would benefit from immunotherapy. Pembrolizumab treatment was initiated in February 2021, but she managed to receive only one cycle due to medication availability. Subsequently, the treatment was switched to nivolumab starting in February 2021.
In June 2021, the patient consulted the emergency department due to inflammatory changes in the lower limbs and bilateral edema. A magnetic resonance imaging was conducted, which reported a solid tubular-shaped lesion with lobulated contours, measuring 75 mm in its largest diameter, with intermediate signal in T2-weighted sequences, exhibiting restriction in diffusion-weighted sequences and heterogeneous enhancement post-contrast. This lesion was located adjacent to the right ischiopubic ramus and was associated with adenopathies containing heterogeneous material, likely necrotic, in the right inguinal region (25 mm in diameter). Additionally, she had a known neoplastic lesion in the left retroperitoneum, with significant fibrotic changes and proximal urethral prominence.
The patient was considered to be experiencing disease progression; therefore, she was again presented in a tumor board meeting, where it was decided to initiate treatment with cetuximab as monotherapy in August 2021, given the expression of epidermal growth factor receptor (EGFR) in this type of tumor. The initial dose was set at 400 mg/m², followed by 250 mg/m² every two weeks until unacceptable toxicity occurred.
During the follow-up, the patient demonstrated adequate tolerance to the treatment, with no need for dose adjustments; however, due to the patient’s overall condition, irinotecan was not added to the regimen. No unacceptable toxicity or need for dose adjustment has been reported to date. A PET-CT re-evaluation was conducted in February 2022, six months after the initiation of treatment, which showed evidence of a complete response with no metabolically active lesions in the abdominal and pelvic regions. The management continued with a second re-evaluation PET-CT in February 2023(Figure 3), which indicated no evidence of metabolic activity suggesting residual or persistent tumor and clinically noted improvement in pelvic symptoms, limb edema, and general symptoms after more than 18 months of treatment with cetuximab.
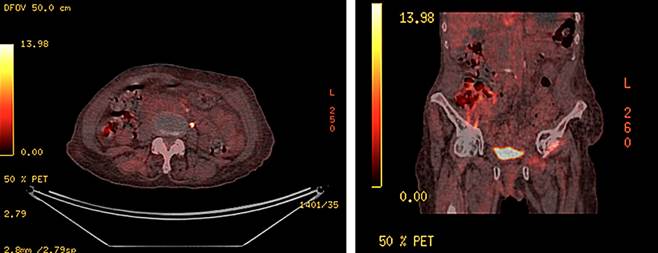
Source: Author’s File.
Figure 3 PET re-evaluation after 18 months of treatment showing sustained complete response.
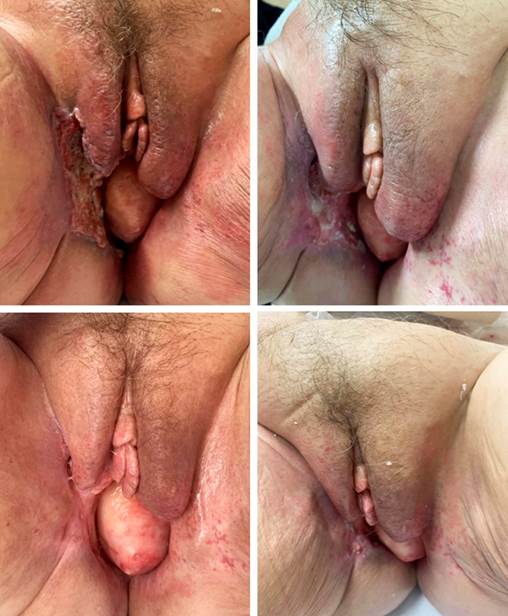
Currently, the patient is under oncology follow-up with maintained response, reduction of inflammatory changes, and adequate healing in the genital region (Figure 4), with acceptable toxicity and good symptom control.
Discussion
This report presents the case of a patient with chemotherapy-resistant squamous cell carcinoma of the anal canal, with a pathological report positive for PD-L1 by CPS, who, after multiple lines of management and surgical treatment, was determined to receive immunotherapy without achieving the expected responses supported by the relevant studies.
The literature supports the use of cetuximab in tumors with overexpression of the EGFR receptor in scenarios such as squamous cell carcinomas of the head and neck and lung, among others, with promising responses. Furthermore, universal expression of EGFR is known in anal squamous cell carcinoma, as well as the absence of KRAS gene mutations, making the use of this medication plausible in anal squamous cell carcinoma4.
Current studies of systemic chemotherapy based on platinum, fluoropyrimidine, and mitomycin show overall survival rates between 5 and 21 months2,4. The efficacy of cetuximab as monotherapy or in combination with chemotherapy or immunotherapy presents variable results in case reports and retrospective case series, with progression-free survival of 5, 7, 8, and even 10 months5-8. A retrospective case series involving 17 patients who received cetuximab as a second or third line of treatment for metastatic disease reported that 35% of patients experienced a reduction in tumor size, 41% had disease progression, and 24% had stable disease, with no patients achieving a complete response. Among the patients who achieved a partial response or stable disease, the median overall survival was 33.8 months, and the median progression-free survival was 12.7 months9.
In the current case, after several lines of management without adequate responses and with rapid progressions, a sustained complete response has been achieved for more than 18 months, with an appropriate quality of life, proper healing, and control of symptoms related to the primary tumor. This result even exceeds the findings of overall survival and progression-free survival in other studies that use targeted therapy either as a single agent or in combination, such as in the CARACAS trial, where patients who received the combination of avelumab and cetuximab achieved a median overall survival of 12.8 months and a median progression-free survival of two months after a median follow-up of 26.7 months10.











 text in
text in