Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Agronomía Colombiana
Print version ISSN 0120-9965
Agron. colomb. vol.34 no.1 Bogotá Jan./Apr. 2016
https://doi.org/10.15446/agron.colomb.v34n1.53325
Doi: 10.15446/agron.colomb.v34n1.53325
Degradation and thermodynamic adsorption process of carbofuran and oxadicyl in a Colombian agricultural soil profile
Degradación y termodinámica del proceso de adsorción del carbofurano y oxadicil en un perfil de suelo agrícola de Colombia
Carmen S. Mosquera-Vivas1, Nelson Obregon-Neira2, Raúl E. Celis-Ossa2, Jairo A. Guerrero-Dallos1, and Carlos A. González-Murillo2
1 Department of Chemistry, Faculty of Science, Universidad Nacional de Colombia. Bogota (Colombia). csmosquerav@unal.edu.co
2 Department of Civil and Agricultural Engineering, Faculty of Engineering, Universidad Nacional de Colombia. Bogota (Colombia)
Received for publication: 1 October, 2015. Accepted for publication: 28 March, 2016
ABSTRACT
Carbofuran and oxadixyl pesticides are used in Colombia to control pests and fungi, but their mobility through the soil profile is poorly understood. This study showed degradation and adsorption processes of these compounds in a Melanudands soil (0-100 cm) from Colombia using laboratory incubation and the batch equilibrium methods. First-order kinetic models indicated that the degradation rates of carbofuran (0.013-0.006 day-1) and oxadixyl (0.013-0.008 day-1) decreased at deeper soil layers, suggesting that the pesticides were more persistent in the sub-surface (60-100 cm) than in the surface layers (0-40 cm). The thermodynamic approach showed that the adsorption of both pesticides was similar, an exothermic and spontaneous process. The carbofuran and oxadixyl coefficient of distribution (5.8-0.3 L kg-1) and the percentage of adsorption (71.2-11.3%) were very similar in the surface layers (0-40 cm) and decreased with the soil depth. The organic carbon (OC) and clay content showed a positive correlation with the pesticide adsorption throughout the soil profile; therefore, mathematical equations were developed from multiple linear regression models for these soil properties and initial concentration. The equations were important to the estimation of the mobility of the compounds using leaching models under laboratory and field conditions.
Key words: pesticide persistence, soil pollution, sorption, chemical degradation, forecasting.
RESUMEN
El carbofurano y oxadicil se aplican en diferentes cultivos en Colombia, pero su transporte a través del perfil del suelo se ha estudiado muy poco. Se evaluó la degradación y la adsorción de ambos plaguicidas en un suelo Melanudands (0-100 cm) mediante el método indirecto e incubaciones bajo condiciones de laboratorio. Las tasas de degradación del carbofurano (0,013- 0,006 día-1) y oxadicil (0,013-0,008 día-1) disminuyeron en las capas más profundas del perfil; lo cual indica que los plaguicidas fueron más persistentes en las capas sub-superficiales (60- 100 cm) que en las capas superficiales (0-40 cm). La adsorción fue un proceso exotérmico y espontáneo. Los coeficientes de distribución (5,8-0,3 L kg-1) y los porcentajes de adsorción (71,2-11,3%) de ambos plaguicidas fueron muy similares en los primeros 40 cm y disminuyeron con la profundidad. Con modelos de regresión lineal múltiple entre la adsorción de los plaguicidas y el contenido de carbón orgánico (CO), las arcillas y la concentración inicial, se obtuvieron ecuaciones matemáticas, las cuales muestran como el CO y las arcillas controlan el transporte de ambos pesticidas a través del perfil. Estas ecuaciones son útiles para estimar el transporte del carbofurano y oxadicil en el suelo.
Palabras clave: persistencia de los plaguicidas, contaminación del suelo, sorción, degradación química, técnicas de predicción.
Introduction
Pesticides are chemicals used to control insects, weeds and/or endemic diseases in order to enhance food production and to protect forests, plantations and fibers (Ecobichon, 2001; Mamy and Barriuso, 2007; Sattler et al., 2007). Car- bofuran is an insecticide banned in Europe and the USA (PAN, 2015; EPA, 2015); in spite of this fact, Latin American and Asian farmers have been using it regularly to control pests in vegetable and fruit crops (Farahani et al., 2007; Valencia et al., 2008; Pimmata et al., 2013). On the other hand, oxadixyl is a fungicide applied to potato, tomato, onion, cut roses and fruit crops (ICA, 2016). The fate of pesticides in the atmosphere-plant-soil system depends on their behavior in the soil. Once the foliar application was carried out, the pesticides moved to the air by volatilization, to the surface water by runoff, to the groundwater by leaching or remained in the soil (adsorption) and were perhaps degraded (Tiryaki and Temur, 2010). The degradation and adsorption processes are critically important to understanding the off-site impact of pesticides applied to field crops in order to protect the environment (Gebre- mariam et al., 2012).
Pesticide degradation rates in soils were dependent upon: the soil type, microorganisms, pesticide type, soil temperature, soil water content, and light irradiation (Shelton and Parkin, 1991; Tariq et al., 2006; Bermúdez-Couso et al., 2013; Pimmata et al., 2013; Dhanasekara et al., 2015). For instance, the natural pyrite degraded nearly 40% of the carbofuran within 100 h (Dhanasekara et al., 2015) and the degradation of the insecticide in the soils with indigenous microorganisms was more rapid that in the sterile soils (Pimmata et al., 2013); although, Tariq et al. (2006) found similar behaviors for sterile and non-sterile soils.
Soil adsorption was the main factor that was responsible for the carbofuran dissipation under darkness (Bermúdez- Couso et al., 2013). Adsorption is the net accumulation of a substance at the interface between a solid phase and an aqueous solution phase (Sposito, 1989; Delle, 2001). The physical and chemical soil properties affect the amount of the chemical adsorbed on the soil solid surface. The OC content, the clay content, the cation exchange capacity (CEC) and the pH seemed to control the adsorption of carbofuran in both the tropical and temperate soils (Delle, 2001; Gupta et al., 2006; Krishna and Philip, 2008; Valencia et al., 2008; Singh and Srivastava, 2009; Bermúdez-Couso et al., 2012). In regards to oxadixyl degradation and ad- sorption, the only available data are those provided by the "pesticide properties database" (PPDB, 2015) for temper- ate soils. Aldana et al. (2011) showed that oxadixyl was a mobile fungicide in soil columns at a 0-30 cm depth and it might be leached through the soil profile. In the under- standing of the adsorption equilibrium between the solid (adsorbent) and liquid phases of soils, thermodynamics play a fundamental role. To have significant adsorption of a chemical in soils, the free energy of adsorption (ΔGads) must be negative and since (ΔGads) = (ΔHads)-T(ΔSads), this requires an enthalpy of adsorption (ΔHads) negative that adsorption entropy (ΔSads). The entropy of adsorption (ΔSads) must be negative due a rotational freedom of the adsorbed substance that is less than the liquid phase substance (Ruthven, 1984).
Most degradation and adsorption studies of carbofuran and oxadixyl have focused on the topsoil layer (Shelton and Parkin, 1991; Tariq et al., 2006; Farahani et al., 2007; Valencia et al., 2008; Aldana et al., 2011; Bermúdez-Couso et al., 2012; Pimmata et al., 2013; Martínez-Cordón et al., 2015). How these pesticides behave throughout the soil profile is poorly understood. Therefore, the aim of this study was to assess the degradation and adsorption of the pesticides carbofuran and oxadixyl (commonly used in Colombia) in one Melanudand soil as a function of soil depth (0-100 cm).
Materials and methods
Soils
Five soil layers (Melanudands), S1 to S5, acquired at different vertical depths in the soil profile (S1: 0-20 cm, S2: 20-40cm, S3: 40-60 cm, S4: 60-80 cm and S5: 80-100 cm), were used in this study. The soil layers were taken in an agricultural landscape in the region of Tenjo-Cundinamarca (Colombia), located at 2,595 m a.s.l. These layers were air- dried and sieved through a US standard sieve with 2.38 mm mesh openings (number 8) for adsorption experiments; whereas, the soil layers for degradation study were kept at field water content. The physical and chemical properties of the soil are summarized in Tab. 1.
Pesticides
Carbofuran [(2,2-dimethyl-3H-1-benzofuran-7-yl) N- methylcarbamate] and oxadixyl [N-(2,6-dimethylphenyl)- 2-methoxy-N-(2-oxo-1,3-oxazolidin-3-yl) acetamide] pesticides (≥ 98% purity) were provided by Dr. Ehrenstorfer GmbH (Augsburg, Germany).
Degradation process
The pesticide degradation was studied with laboratory incubation experiments (Mamy and Barriuso, 2007; Mosquera-Vivas et al., 2010). Ten g (10.00) of dry soil (S1- S5) were placed in a 50 mL centrifuge tube and spiked with 0.250 mL of the pesticide mixture. Carbofuran and oxadixyl were added at reported field doses of a.i. 0.31 and 1.0 kg ha-1, respectively. These samples were then inserted in 500 mL jars containing 20 mL of 2 M NaOH (on top) and a sealed vial with 10 mL of distillated water. The samples were incubated under unsaturated field moisture conditions (Tab. 1) at 18ºC in the dark for 102 d. After incubation times of 0, 1, 3, 7, 14, 31, 45, 60, 76 and 102 d, two soil samples from each depth were dried on foil containers at room temperature (18°C) for 3 d. The samples were then placed in centrifuge tubes, spiked with PCB 103 (surrogate compound) and extracted with 20 mL of ethyl acetate and shaken for 15 min. Na2SO4 (5.0 g) and NaHCO3 (1.7 g) were then added and the samples were shaken for 15 min again. The samples were centrifuged at 7,500 rpm for 15 min and the organic supernatants (10 mL) concentrated to 2 mL. An aliquot of PCB 52 (internal standard) was then added before injecting the samples into a CG-MS system. The limit of detection (LOD) was 0.008 μg g-1 for carbofuran and 0.028 μg g-1 for oxadixyl. The limits of quantification (LOQ) were 0.020 μg g-1 and 0.118 μg g-1 for carbofuran and oxadixyl, respectively. The recovery of the pesticides was 103.3±7.8% for carbofuran and 79.1±5.9% for oxadixyl. For instance, the recovery of PCB 103 was 91.9% with a RSD of 1.4%.
The first-order kinetic model was fitted to obtain the degradation rate (k) and the half-life (t1/2) of the two pesticides (Eq. 1 and 2):

where Ct is the pesticide concentration at time t and C0 is the initial concentration of the pesticide.
Adsorption process
The adsorption process was obtained by using the batch equilibrium method (Mamy and Barriuso, 2007; Langeron et al., 2014). All of the pesticides were studied together in a mixture solution according to the field data. Five test substances were prepared in 0.01 M CaCl2. The concentration ranges were 0.13 - 0.40 mg L-1 for carbofuran and 0.49 - 2.16 mg L-1 for oxadixyl. All of the experiments were executed with samples of pesticides in 0.01 M CaCl2 and blanks with soil in 0.01 M CaCl2.
Ten g (10.00) of dry soil (S1-S5) were placed in 50-mL centrifuge tubes and a 20.0 mL aliquot of the mixture was added to each tube. The soil-water slurry was shaken for 24 h at 20°C and centrifuged at 7,000 rpm for 30 min. The supernatant was removed, weighed and extracted by liquid-liquid extraction. The supernatant was spiked with PCB 103 and the extraction was carried out with 20.0 mL of ethyl acetate and shaken for 15 min. NaCl was added and the sample was shaken again for an additional 15 min before sonication for 15 min. The organic phase was concentrated to 1 mL. An aliquot of PCB 52 was added to the samples before injection into a CG-MS system. The pesticide and PCB 103 recoveries by liquid-liquid extraction were 89.5±8.5% for carbofuran, 81.9±5.8% for oxadixyl and 84.9±7.1% for PCB 103.
The coefficient of distribution (kd) was calculated as the average of the subsamples for each layer (Eq. 3):

Ceq is the equilibrium concentration of the pesticide (mg L-1) in the soil solution and n is the number of the data.
Finally, the soil organic carbon-water partitioning coeffi- cient, kOC, was obtained by normalizing the kd coefficients to the OC content (Eq. 4):

Pedotransfer functions (PTF) were used to estimate the kd values of the pesticides from the OC content, clay content and pH (Weber et al., 2004; Singh et al., 2014). Weber et al. (2004) used 13 data points to get a linear model between linear model between the kd of carbofuran as the carbofuran as a dependent variable, and the OC and the clay content as independent variables. In order to obtain multiple linear regression models for both pesticides, we performed a multicollinearity test using Pearson correlation coefficients and the variance inflation factor (VIF) with all of the data (n = 22) throughout the soil profile. Linear parameters were obtained for the combination of the OC content, the clay content and the initial concentration of the substances, where VIF was used to select the best linear equations.
Pesticide quantification
The quantification of the pesticides in the soil and aqueous phases was performed using an Agilent (Santa Clara, CA) model 7890A gas chromatography coupled to a 5975C mass spectrometer detector. The chromatography system was equipped with a multimode inlet, autosampler and HP5- MS (30 m x 250 μm x 0.25 μm) column. The carrier gas used was helium and the injection volume was 4 μL with the solvent vent mode. The MS transfer line was kept at 280ºC and the quantification was performed using selected ion monitoring (SIM).
Thermodynamic approach
Adsorption enthalpy (ΔHads) is given by (Goss and Schwarzenbach, 1999):

where,  is the coefficient of distribution at Tr (reference temperature = 293 K) and
is the coefficient of distribution at Tr (reference temperature = 293 K) and  is the average specific surface of the soil, which can be estimated as (Vighi and Di Guardo, 1995)
is the average specific surface of the soil, which can be estimated as (Vighi and Di Guardo, 1995)

where foc is the soil carbon fraction, fcl is the soil clay fraction, fst is the soil silt fraction and fsd is the soil sand fraction.
Adsorption free energy at Tr is calculated as:

where R is the gas constant and kd is:

where [Pads] is the equilibrium concentration of the pesticide in the soil solid (mole L-1), [Psln] is the equilibrium concentration of the pesticide in the soil solution (mole L-1) and ρp is the density of the particles (kg L-1).
Adsorption entropy can be calculated according to (Ruthven, 1984)

ΔSads can be used to understand the adsorption process described in the reaction below:

where S(s) is the soil solid, P(sln) is the pesticide in the soil solution and S – P(ads) is the pesticide adsorbed in the soil solids.
Results and discussion
Degradation process
The first-order kinetic models for carbofuran and oxadixyl in the soil profile are shown in Fig. 1. The degradation rate (k) and the half-life time (t1/2) calculated from this model are given in Tab. 2. Carbofuran was completely degraded in S1 after 7 d of incubation. In S2-S4, the model fitted accurately to data with coefficients of determination (R2)>0.75 and the null hypothesis (H0: k = 0 and C0 = 0) were rejected at a 95% confidence level. The k values of the insecticide (0.006-0.013 d-1) decreased from S2 to S3 and remained constant in S4, which may be explained by the change of microbial activity throughout the soil profile. Soil microorganisms play an important role in the pesticide degradation process (Mosquera-Vivas et al., 2010; Pimmata et al., 2013) and, in our soil, the amount of CO2 evolved during the microbial respiration was found to decrease in S1-S3, after which it was constant (Fig. 2); accordingly, the decreasing of microbial activity allowed the k values to decrease. It is seemed with k value of carbofuran in S5 where the concentration remained unchanged; hence, the first-order kinetic showed a poor fit, as was expected. The degradation rates of 2,4-D and atrazine decreased with the increasing soil depth, which was due to the microbiota decrease through the soil profile (Kruger et al., 1993; Veeh et al., 1996). Microbial degradation is an important degradation pathway of carbofuran in neutral and acid soils (Evert, 1991; Pimmata et al., 2013). t1/2 values of the insecticide increased at deeper soil depths. t1/2 for soil layers S1 and S2 (10.5-53.3 d-1) is within the range reported by Pimmata et al. (2013) and Tariq et al. (2006) for Pakistan and Thailand soils (1.6-69.3 d-1); although, t1/2 in soil layers at a depth of 40-100 cm is higher than the former ones, suggesting the insecticide was more persistent at the deeper layers in the studied soil profile.

The first-order kinetic fit well to oxadixyl degradation data with R2 values between 0.83 and 0.95, and the null hypothesis (H0: k = 0 and C0 = 0) was rejected at a 95% confidence level, except in S5 (Fig. 1 and Tab. 2). k values of the oxadixyl were similar in S1-S3 (0.012-0.013 d-1) and slightly decreased to 0.008 d-1 in S4. This behavior might be explained by the fact that the biotic process occurs mainly in S1 and S2, where the amount of CO2 evolved during the microbial respiration was higher than in S3 and S4 (Fig. 2). In layers S3 and S4, the abiotic process probably degraded the fungicide. It seems that hydrolysis controled the degradation process in layers at a depth of 40-80 cm because the oxadixyl showed a heterocyclic ring system, which is liable to hydrolysis (Roberts and Hutson, 1998). In contrast to the carbofuran, the oxadixyl was degraded to nearly 10.0% of its maximum concentration during 102 d of incubation in S5, confirming the hydrolysis reaction as well. The t1/2 of the oxadixyl also increased at the deeper soil layers (Tab. 2). The t1/2 of the fungicide in S1-S3 was smaller than the values published in the pesticide properties database in topsoils from temperate regions (PAN, 2015; PPDB, 2015), showing that the oxadixyl degraded faster under tropical conditions and it was more persistent at deeper soils layers, i.e. in S4 and S5.
Adsorption process
The kd and koc values and the adsorption percentage for the carbofuran and oxadixyl in soil layers S1-S5 are shown in Fig.3. kd values and the adsorption percentage for both pesticides were higher in S1-S2 than in S3-S5. Although the carbofuran and oxadixyl showed an aromatic ring, a heterocyclic ring and oxygen atoms, which are liable to adsorb the pesticides in our soil with the same physical interactions. The adsorption behavior for the carbofuran and oxadixyl through the soil profile can be explained by the decrease in the OC content, clay content, CEC and ECEC. The kd values revealed a positive correlation with the OC content (r = 0,97 and 0.92), clay (r = 0.97 an d 0.93), CEC (r = 0.99 and 0.99) and ECEC (r = 0.88 and 0.95). Similar trends have been published by Delle (2001), Valencia et al. (2008), Singh and Srivastava (2009), Bermúdez-Couso et al. (2012), and Pimmata et al. (2013). Positive correlation between kd, ECEC and OC showed the role of the oxygen atoms in the retention capacity of both pesticides in the soils. The oxygen atoms attracted electron density (electronegativity) and the negative net of the soil repelled the pesticides; as a consequence, the kd values were very small (0.3-5.8 L kg-1). The decreasing values of carbofuran and oxadixyl adsorption with soil depth might be explained by the decreased concentration of the divalent cations, Ca2+ and Mg2+ (Tab. 1). These cations form soil-divalent cation- pesticides linkages.

The koc values of the insecticide and the fungicide in S1-S5 varied from 40.3 to 13.7 L kg-1 and from 55.6 to 11.1 L kg-1, respectively. The carbofuran range of the koc at a depth of 0-60 cm (Fig. 3) was within the one reported for Colombian soils (Valencia et al., 2008); however, all of the koc values were lower than that found for tropical soils from India and Malaysia. (Farahani et al., 2007; Krishna and Philip, 2008; Singh and Srivastava, 2009), it might suggest the adsorption of the insecticide was affected by the soil genesis. In contrast, the oxadixyl range of the koc at a depth of 0-40 cm soils; although, the range of the Koc at a depth of 40-100 cm was within the range published in the literature (PAN, 2015; EPA, 2015; Martínez-Cordón et al., 2015), indicating that the adsorption of the fungicide in our topsoil (0-40 cm) was higher than those found in other topsoils. This behavior could result from the composition of the soil organic matter throughout the profile.
The multiple linear regression models for the carbofuran and oxadixyl throughout the soil profile are summarized in Eqs 13-18:
Carbofuran:

Oxadixyl:

These models can be used to calculate the coefficient of distribution and/or the adsorption percentage using the OC and clay content. To calculate kd and the percentage of adsorption from PTF may reduce the cost and time in chemical analysis to predict the mobility of carbofuran and oxadixyl in Colombia soils. Furthermore, the models confirmed the relationship between the adsorption of pesticides and the OC and clay content.
The enthalpy, entropy and free energy values of the adsorption of the pesticides in layers S1-S5 are summarized in Tab. 3. The Fig. 4 shows the adsorption free energy of the carbofuran and oxadixyl throughout the soil profile. All of the thermodynamic approaches were negative, except the free energy for both pesticide in soil layer S5, suggesting that the adsorption of the pesticides in our soils was exothermic and spontaneous and the rotational freedom of the adsorbed chemicals was less than the liquid phase chemicals (Ruthven, 1984). Furthermore, ∆Gads was similar for the carbofuran and oxadixyl throughout the soil profile (Tab. 3 and Fig. 4). Negative free energy (∆Gads) has also been published for the adsorption of carbofuran in India soils at a depth of 0-30 cm (Singh and Srivastava, 2009). Low values of free energy indicate that the adsorption of the pesticides in soil solids is often promoted by weak physical forces (Weber, 1993; Singh and Srivastava, 2009).
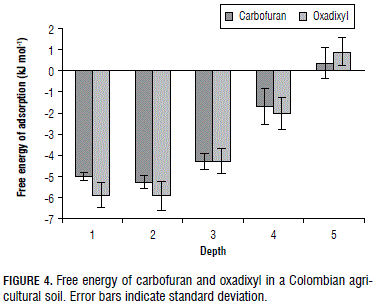
For instance, the carbofuran and oxadixyl were adsorbed less than the dimethomorph (data not shown) and this is in accord with their chemical structure. Dimethomorph shows two aromatic ring system and chlorine atom, while carbofuran and oxadixyl exhibit one aromatic ring system without a chlorine atom. The aromatic rings increase π-π stacking interactions and the chlorine atom promotes hydrophobic interactions between pesticide and aromatic components of soil organic matter.
Positive free energy for the insecticide and fungicide in S5 showed that the adsorption of both pesticides was not a spontaneous process, i.e. carbofuran and oxadixyl prefer the soil aqueous solution.
Conclusion
The high microbial activity played a key role reducing the transport of both pesticides at a depth of 0-40 cm. If carbofuran and oxadixyl reach deeper soil layers (60-100 cm), they might constitute a risk for groundwater pollution due to their persistence and mobility, as shown above.
Acknowledgements
This study was funded by the Swiss National Science Foundation (SNSF) and the Swiss Agency for Development and Cooperation (SDC) [Project number: IZ70Z0_124080]. Furthermore, the authors would like to thank Colciencias (Project number: 528) for economical support and Asocolflores (Direction of Environmental Issues) for technical support.
Literature cited
Aldana, M., R. de Prado, and M.J. Martínez. 2011. Leaching of oxadyxil and tebuconazole in Colombian soil. Commun. Agric. Appl. Biol. Sci. 76, 909-914. [ Links ]
Bermúdez-Couso, A., D. Fernández-Calviño, I. Rodríguez-Salgado, J.C. Nóvoa-Muñoz, and M. Arias-Estévez. 2012. Comparison of batch, stirred flow chamber, and column experiments to study adsorption, desorption and transport of carbofuran within two acidic soils. Chemosphere 88, 106-112. Doi: 10.1016/j.chemosphere.2012.02.078 [ Links ]
Bermúdez-Couso, A., J.C. Nóvoa-Muñoz, M. Arias-Estévez, and D. Fernández-Calviño. 2013. Influence of different abiotic and biotic factors on the metalaxyl and carbofuran dissipation. Chemosphere 90, 2526-2533. Doi: 10.1016/j.chemosphere.2012.10.090 [ Links ]
Delle S., A. 2001. Factors affecting sorption of organic compounds in natural sorbent/water systems and sorption coefficients for selected pollutants. A review. J. Phys. Chem. Ref. Data 30, 187. Doi: 10.1063/1.1347984 [ Links ]
Dhanasekara, S.A.K.M., A.N.B. Attanayake, A.C. Herath, N. Nanayakkara, A. Senaratne, S.P. Indrarathne, and R. Weerasooriya. 2015. Partial degradation of carbofuran by natural pyrite. Environ. Nanotech. Monit. Manage. 4, 51-57. Doi: 10.1016/j.enmm.2015.07.002 [ Links ]
Ecobichon, D.J. 2001. Pesticide used in developing countries. Toxicology 160, 27-33. Doi: 10.1016/S0300-483X(00)00452-2 [ Links ]
EPA, Environmental Protection Agency. 2015. Carbofuran cancellation process. In: www.epa.gov/oppsrrd1/reregistration/carbofuran/carbofuran_noic.htm; consulted: August, 2015. [ Links ]
Evert, S. 1991. Environmental fate of carbofuran. In: Department of Pesticide Regulation, http://www.cdpr.ca.gov/docs/emon/pubs/fatememo/carbofuran.pdf; consulted: April, 2016. [ Links ]
Farahani, G.H.N., Z. Zakaria, A. Kuntom, D. Omar, and B.S. Ismail. 2007. Adsorption and desorption of carbofuran in Malaysian soils. Adv. Environ. Biol. 1, 20-26. [ Links ]
Gebremariam, S.Y., M.W. Beutel, D.R. Yonge, M. Flury, and J.B. Harsh. 2012. Adsorption and desorption of chlorpyrifos to soils and sediments. Rev. Environ. Contam. Toxicol. 215, 123-175. Doi: 10.1007/978-1-4614-1463-6_3 [ Links ]
Goss, K.U. and R.P. Schwarzenbach. 1999. Empirical prediction of heats of vaporization and heats of adsorption of organic compounds. Environ. Sci. Technol. 33, 3390-3393. Doi: 10.1021/es980812j [ Links ]
Gupta, V.K., I. Ali, Suhas, and V.K. Saini. 2006. Adsorption of 2,4-D and carbofuran pesticides using fertilizer and steel industry wastes. J. Colloid Interf. Sci. 299, 556-563. Doi: 10.1016/j.jcis.2006.02.017 [ Links ]
ICA, Instituto Colombiano de Agricultura. 2016. Registro nacionales-marzo de 2016. In:, www.ica.gov.co/getdoc/d3612ebf-a5a6-4702-8d4b-8427c1cdaeb1/REGISTROS-NACIONALES-PQUA-15-04-09.aspx; consulted: April, 2016. [ Links ]
Krishna, K.R. and L. Philip. 2008. Adsorption and desorption characteristics of lindane, carbofuran and methyl parathion on various Indian soils. J. Hazard. Mater. 160, 559-567. Doi: 10.1016/j.jhazmat.2008.03.107 [ Links ]
Kruger, E.L., L. Somasundaram, J.R. Coast and R.S. Kanwar. 1993. Persistence and degradation of [14C]atrazine and [14C]deisopropylatrazine as affected by soil depth and moisture conditions. Environ. Toxicology Chem. 12, 1959-1967. Doi: 10.1002/etc.5620121102 [ Links ]
Langeron, J., A. Blondel, S. Sayen, E. Hénon, M. Couderchet, and E. Guillon. 2014. Molecular properties affecting the adsorption coefficient of pesticides from various chemical families. Environ. Sci. Pollut. Res. 21, 9727-9741. Doi: 10.1007/s11356-014-2916-6 [ Links ]
Mamy, L. and E. Barriuso. 2007. Desorption and time-dependent sorption of herbicides in soils. Eur. J. Soil Sci. 58, 174-187. Doi: 10.1111/j.1365-2389.2006.00822.x [ Links ]
Martínez-Cordón, M.J., M.I. Aldana-Castañeda, and J.A. Guerrero-Dallos. 2015. Modelación matemática del transporte de oxadixyl en suelos de cultivo de cebolla. Rev. Ambient. Água 10, 327-337. Doi: http://dx.doi.org/10.4136/ambi-agua.1565 [ Links ]
Mosquera-Vivas, C.S., M.J. Martínez-Cordón, and J.A. Guerreo-Dallos. 2010. 14C tebuconazole degradation in Colombian soils. Commun. Agric. Appl. Biol. Sci. 75, 173-181. [ Links ]
PAN. 2015. Which pesticides are banned in Europe? In: PAN UK Food & Fairness, www.pan-europe.info/Resources/Links/Banned_in_the_EU.pdf; consulted: April, 2016. [ Links ]
Pimmata, P., A. Reungsang, and P. Plangklang. 2013. Comparative bioremediation of carbofuran contaminated soil by natural attenuation, bioaugmentation and biostimulation. Int. Biodeter. Biodegr. 85, 196-204. Doi: 10.1016/j.ibiod.2013.07.009 [ Links ]
PPDB. 2015. The PPDB pesticide properties Database. In: http://sitem.herts.ac.uk/aeru/ppdb/en/; consulted: April, 2015. [ Links ]
Ruthven, D.M. 1984. Physical adsorption and the characterization of porous adsorbents. pp. 29-85. In: Ruthven, D.M. (ed.). Principles of adsorption and adsorption process. John Wiley & Sons. New York, NY. [ Links ]
Roberts, T. and D.H. Hutson. 1998. Metabolic pathway of agrochemicals: insecticide and Fungicide, Part 2. The Royal Society of Chemistry, Cambridge, UK. [ Links ]
Sattler, C., H. Kächele, and G. Verch. 2007. Assessing the intensity of pesticide use in agriculture. Agric. Ecos. Environ. 119, 299-304. Doi: 10.1016/j.agee.2006.07.017 [ Links ]
Shelton, D.R. and T.B. Parkin. 1991. Effect of moisture on sorption and biodegradation of carbofuran in soil. J. Agric. Food Chem. 39, 2063-2068. Doi: 10.1021/jf00011a036 [ Links ]
Singh, R.P. and G. Srivastava. 2009. Adsorption and movement of carbofuran in four different soils varying in physical and chemical properties. Adsorpt. Sci. Technol. 27, 193-203. Doi: 10.1260/026361709789625270 [ Links ]
Singh, B., A. Farenhorst, J. Gaultier, D. Pennock, D. Degenhardt, and R. McQueen. 2014. Soil characteristics and herbicide sorption coefficients in 140 soil profiles of two irregular undulating to hummocky terrains of western Canada. Geoderma 232-234, 107-116. Doi: 10.1016/j.geoderma.2014.05.003 [ Links ]
Sposito, G. 1989. The chemistry of soils. Oxford University Press, Oxford, UK. [ Links ]
Tariq, M.I., S. Shahzad Afzal, and I. Hussain. 2006. Degradation and persistence of cotton pesticides in sandy loam soils from Punjab, Pakistan. Environ. Res. 100, 184-196. Doi: 10.1016/j.envres.2005.05.002 [ Links ]
Tiryaki, O. and C. Temur. 2010. The fate of pesticide in the environment. J. Biol. Environ. Sci. 4, 29-38. [ Links ]
Valencia, E.M., J.A. Guerrero, A. de Yunda, and M.J. Martínez. 2008. Evaluación de la adsorción-desorción de 14C-carbofuran y Furadan 3SC® en tres suelos de Cundinamarca (Colombia). Rev. Colomb. Quim. 37, 79-91. [ Links ]
Veeh, R.H., W.P. Inskeep, and A.K. Camper. 1996. Soil depth and temperature effects on microbial degradation of 2,4-D. J. Environ. Qual. 25, 5-12. Doi: 10.2134/jeq1996.00472425002500010002x [ Links ]
Vighi, M. and A. Di Guardo. 1995. Predictive approaches for the evaluation of pesticide exposure. pp. 73-100. In: Vighi, M. and E. Funari (eds.). Pesticide risk in groundwater. CRC Press, Boca Raton, FL. [ Links ]
Weber, J.B. 1993. Ionization and sorption of fomesafen and atrazine by soils and soil constituents. Pest Manag. Sci. 39, 31-38. Doi: 10.1002/ps.2780390105 [ Links ]
Weber, J.B., G.G. Wilkerson, and C.F. Reinhardt. 2004. Calculating pesticide sorption coefficients (kd) using selected soil properties. Chemosphere 55, 157-166. Doi: 10.1016/j.chemosphere.2003.10.049 [ Links ]














