Introduction
Black oats (Avena strigosa Schreb.) is a winter grass, with good adaptability to soils with low fertility (Fontaneli et al., 2012). It is used as a cover crop (Wolschick et al, 2016) and as a forage crop singly or in association with other crops, showing good forage yield and higher weight gain for cattle production (Ferrazza et al., 2013; Lupatini et al, 2013).
The use of high-quality seeds can significantly influence black oats performance in the field, reflecting, for example, higher dry matter yield and leaf area index (Schuch et al, 2008). Seed quality is characterized by physical quality, measured mainly by moisture content, 1000-seed weight, and hectoliter weight, and by physiological performance measured by the germination and vigor tests (Marcos Filho, 2015). However, seed quality can be influenced by drying and storage operations, as verified by white oat seeds (Oliveira et al., 2010) and wheat seeds (Scariot et al, 2018).
Black oat seeds are orthodox, i.e. they are long-lived and dessicant-tolerant and so are tolerant of a reduction in moisture content. As dessicant-tolerant seeds, drying allows them to be stored with better quality and for a longer time. Among the main factors to be considered during the seed drying process is the air temperature. The use of elevated drying air temperatures can result in damage such as cracks and fissures, reserve denaturation, cell membrane damage, germination power loss and even embryo death. Any seed sensitivity to thermal damage can vary according to the plant species, exposure time to heat and drying speed (Garcia et al, 2004; Afrakhteh et al, 2013).
Seed storage aims to minimize quality losses over time. However, during storage there may be changes in seed quality due to deterioration and aging. Such modifications can be influenced by the storage conditions, mainly in relation to the temperature and relative humidity, but also by the initial physical and physiological conditions of the seeds. Seeds that are damaged or degraded at the beginning of storage are more fragile and, consequently, lose quality more quickly over storage time (McDonald, 1999; Deliberali et al, 2010). Damages caused by the drying temperature can be detected immediately after drying or during storage. These kinds of damages are called immediate and latent damages (Afonso Júnior and Corrêa, 2000).
After drying, the seeds were sent to storage. Storage began on December 2, 2016. Seeds were stored in a conventional system (kraft paper bags) and in a natural environment for 180 d. During the experiment, the temperature and relative humidity of the storage location were monitored daily. The data are shown in Fig. 1 in monthly means.
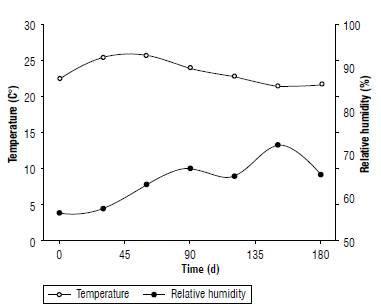
FIGURE 1 Monthly means of temperature and relative humidity of the black oat seeds storage location.
Some studies have highlighted the damage caused by the drying air temperature on the quality of oat seeds (Oliveria et al, 2010). However, there are few (if any) studies on the behavior of dry oat seeds with different drying air temperatures during the storage period. The objective of this study was to evaluate the immediate and latent damages of different drying air temperatures on the physical and physiological quality of black oat seeds.
Materials and methods
This experiment was carried out at the Laboratory of the Agricultural Systems and Sustainable Management Group (MASSA) of the Universidade Federal da Fronteira Sul (UFFS), Erechim, RS. The experiment had a completely randomized design, arranged in a 4x5 factorial scheme (drying air temperatures x storage time) with four replicates.
Black oat seeds, cultivar EMBRAPA 139 (Neblina), were obtained from the experimental area of the UFFS during the 2016 crop season. The crop cultivation was accomplished under a no-tillage system with fertilization and phytosani-tary management, carried out according to the technical suggestions for the crop. Harvesting was performed manually when the seeds contained approximately 25% moisture content. After that the material was subjected to threshing with the aid of a mechanical thresher.
Samples containing 500 g of seeds were packed in kraft paper bags and dried in an oven (Marconi®, model MA033/1080, Piracicaba, SP, Brazil) with forced air circulation (stationary drying) at drying air temperatures of 30°C, 40°C and 50°C until reaching approximately 11% moisture content. Additionally, a drying treatment was tested that alternated the air temperatures, i.e. the seeds were first exposed to a temperature of drying air of 40°C until reaching approximately 16% moisture content and then they were subjected to a temperature of 50°C to reach 11% moisture content.
During seed storage there was a reduction in temperature and an increase in the relative humidity of the air due to the approach of the winter period. The temperatures during seed storage varied between 21.4°C and 26.8°C, with a mean temperature of 23.3°C. The relative humidity showed values between 56% and 72% with an average of 62%.
Laboratory analyses were carried out immediately after drying and later every 45 d, defining five evaluation moments (0, 45, 90, 135, and 180 d). The moisture content, 1000-seed weight, hectoliter weight, electrical conductivity, and germination were analyzed. Seed vigor was determined by the tests of the first germination count, the germination speed index, accelerated ageing and the shoot length.
The moisture content was determined in triplicate for each replicate by the oven method at 105±3°C for 24 h (Ministério da Agricultura, Pecuária e Abastecimento, 2009). The results were expressed as a percentage and wet basis (wb).
The weight of 1000 seeds was determined by weighing eight subsamples of 100 seeds for each replicate, with the results being expressed in grams (Ministério da Agricultura, Pecuária e Abastecimento, 2009). The hectoliter weight was determined with the aid of a hectoliter weight balance (Comag®, Panambi, RS, Brazil), with a quarter liter capacity. Values were obtained in triplicate for each replicate and the results expressed in kg hl-1 (Ministério da Agricultura, Pecuária e Abastecimento, 2009). The values of a 1000-seeds weight and hectoliter weight were adjusted to 13% moisture content.
The germination test was conducted on Germitest paper rolls, previously soaked in distilled water at a ratio 2.5 times their weight and kept in a germinator (Tecnal, model TE-405, Piracicaba, SP, Brazil) at 20±3°C, with a photoperiod of 12 h, without the use of agents to overcome dormancy. Two hundred seeds per replicate were used, distributed in four rolls of 50 seeds that totaled 800 seeds per treatment. The first count was performed on the fifth day after sowing by counting the number of normal seedlings. The final evaluation was carried out on the 10th day after sowing. The results were expressed as a percentage (Ministério da Agricultura, Pecuária e Abastecimento, 2009).
The germination speed index was carried out simultaneously with the germination test, by counting the number of normal germinated seeds per day, from the time of sowing until the fifth day, and the index was determined as proposed by Maguire (1962).
The electrical conductivity was determined by the mass system, with two samples of 50 seeds per repetition, conditioned in a glass beaker, weighed and immersed in 50 ml of distilled water. The containers were kept in B.O.D chambers at a temperature of 25±3°C. Evaluations were carried out 24 h after seed immersion with the aid of a benchtop electrical conductivity meter (Gehaka®, model CG1800, São Paulo, SP, Brazil). Results were expressed as US cm-1 g-1 (Marcos Filho et al, 1987).
In the accelerated ageing test, the seeds were placed in a gerbox containing 50 ml of distilled water, suspended with a grid, and conditioned in a B.O.D chamber at 41°C for 72 h (Marcos Filho et al., 1987). After this stage the test was conducted as described for the germination test, and was carried out on the fifth day after sowing, counting the number of normal seedlings, and expressing the results as percentages.
For the determination of shoot length, the seeds were sown on the same way as described for the germination test.
Eighty seeds were used per replicate, distributed in four rolls of germitest paper containing 20 seeds each, totaling 320 seeds per treatment. On the 10th day after sowing, 10 seedlings of each roll were randomly chosen and measured with a ruler graded in millimeters. The results were expressed in cm (Nakagawa, 1994).
Data were submitted to analysis of variance by the F-test (P≤0.05). The drying temperatures were compared with the Tukey's test (P≤0.05) using the Statistica® 10.0 software. For storage time, regression analysis was applied through Sigma Plot® 10.0 software. The models were selected based on the significance of the model parameters by the application of the t-test (P≤0.05), by the significance of the mathematical model (P≤0.05), by the coefficient of determination (r2≥0.60) and by the biological phenomenon.
Results and discussion
According to the variance analysis performed using the F-test (P≤0.05), there was a significant interaction between the factors drying air temperature and storage time for all variables analyzed.
The values of seed moisture content for the different drying temperatures showed oscillations throughout the storage period, tending to the hygroscopic equilibrium as a function of the relative humidity and the air temperature in the storage environment, which showed relative humidity and average air temperature values of 62% and 23.3°C, respectively. However, no mathematical model showed a satisfactory fit for the observed data (Fig. 2A). Similar results were found by Scariot et al. (2018), who observed oscillations in the moisture content of wheat seeds stored for 240 d under environmental conditions. The 1000-seed weight and hectoliter weight of seeds decreased linearly over the storage time, regardless of the drying temperature used (Fig. 2B and C).
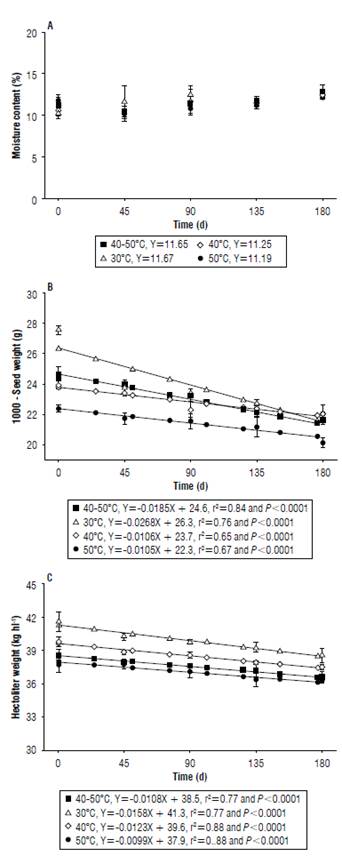
FIGURE 2 A) Moisture content, B) 1000-seed weight, and C) hectoliter weight of black oat seeds cultivar EMBRAPA 139 (Neblina), subjected to different drying temperatures and stored under ambient conditions for 180 days.
The reduction of the 1000-seed weight and hectoliter weight of seeds throughout the storage occurs because of the respiratory process that causes the consumption of reserves and, consequently, the reduction of seed weight and density (Wang et al., 2018). The respiratory rate of seeds during storage can be influenced by several factors: temperature and air relative humidity, as well as thermal damage suffered in drying and pest damages (Ferrari Filho et al, 2012).
Soon after drying, the drying temperature of 30°C provided seeds with greater specific mass and a 1000-seed weight, while lower values were observed with the other temperatures. The lowest values were observed at a temperature of 50°C, indicating immediate damage. Seeds dried at alternating temperatures showed better physical quality when compared to the ones dried only at a temperature of 50°C.
The reduction of the 1000-seed weight throughout storage was more pronounced for the lower temperatures, including the alternating temperatures. This result indicated more latent damage when compared to the temperature of 50°C, that showed a less marked reduction, but with 1000-seed weight values lower than the other temperatures during the entire experiment. For the hectoliter weight, similar behavior was observed for the reduction in the values between the temperatures and in the alternating temperature. This indicated that the latent seed density damage was similar between the tested temperatures. However, at the end of storage the seeds dried at the lower temperatures had a higher specific mass.
These results agreed with Oliveira et al. (2010), who verify the reduction of the 1000-seed weight and hectoliter weight of white oat seeds with the increase of drying air temperature from 50°C.
The reduction in physical quality of the seeds caused by the increase in the temperature of the drying air can also be related to thermal damages like cracks and fissures. For hectoliter weight, the appearance of cracks causes an increase in the seed volume because of the appearance of empty spaces, which leads to density reduction (Eichelberger and Portella, 2003).
Seed germination throughout storage showed similar behavior for the drying temperatures that were studied. The increase in the values was observed for up to 90 d of storage with a subsequent reduction due to the degrading and ageing of seeds (Fig. 3A). Throughout the experiment the highest germination percentages were obtained from the seeds that were dried at a temperature of 30°C.
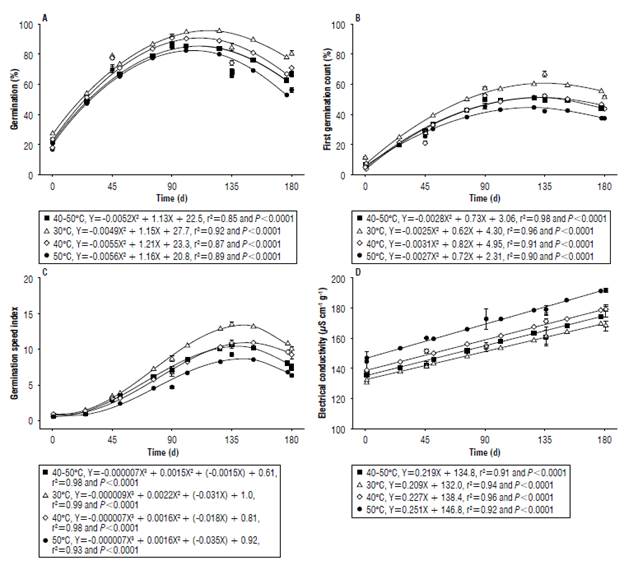
FIGURE 3 A) Germination, B) first germination count, C) germination speed index, and D) electrical conductivity of black oat seeds cultivar EMBRAPA 139 (Neblina), subjected to different drying temperatures and stored under ambient conditions for 180 days.
This described behavior of seeds is characteristic of some species soon after harvesting and includes black oats, due to the process of primary or innate dormancy that is genetically programmed into the mother plant as a function of environmental conditions. Overcoming dormancy can occur over time under conditions of low seed moisture content, culminating in the increase of the germination percentage in storage (Grzybowski et al, 2015).
No immediate damage was observed due to the increase of the drying temperature on seed germination. However, at the end of the storage period, a reduction in seed germination percentage was observed with increasing drying temperature, showing the latent effect of temperature on black oat seed germination. According to Nakagawa et al. (2004), the seed's conservability during storage is related to the factors that define its initial quality, including drying.
The results are in agreement with those obtained by Oliveira et al. (2010) who demonstrated that stationary drying at temperatures of 50°C, 75°C and 110°C resulted in a reduction in white oat seed germination immediately after drying when compared to a control (25°C). However, the authors used higher temperatures than those used in this study, which may have caused immediate damage.
Seed vigor, when analyzed by germination, first germination, and speed index count tests, showed similar behavior throughout the storage for all the drying temperatures, with an increase in the values until approximately 135 d, and a drop until the end the storage period (Fig. 3B and C). This behavior is due to seed dormancy that is overcome with storage time and is reflected in the increased physiological activity of the seed.
Regarding germination, no immediate damage was observed due to the increase in drying temperature on the germination speed index. For the first germination count, a higher percentage of normally-germinated seedlings were observed for the drying temperature of 30°C, indicating a higher vigor. However, during the storage time, lower values were verified for these two variables with the increase in the drying temperature, indicating loss of quality by the latent effect.
Electrical conductivity showed a linear increase according to storage time for all drying temperatures (Fig. 3D). The increase of electrical conductivity in storage is due to the process of deterioration and seed ageing. The destabilization of cell membranes is one of the first events related to these processes that culminate with the increase of solute leaching and electrical conductivity (Mira et al., 2011). These results are similar to those observed by Zucareli et al. (2015) and Scariot et al. (2018) who verified the increase in electrical conductivity of bean and wheat seeds over storage time.
The elevation of drying temperature caused immediate and latent damages in the cellular structure of black oat seeds, since the electrical conductivity values were higher at higher drying temperatures throughout the storage time. This result can be attributed to the fact that the increase in drying air temperature provides rapid removal of water from seeds. This can cause damage to the selective permeability of cell membranes, increasing solute leaching, reflected by higher conductivity values (Corrêa and Afonso, 1999). Botelho et al. (2015) and Scariot et al. (2017a) verified increased electrical conductivity of soybean and bean seeds with an increase in drying air temperature of up to 80°C and 50°C, respectively.
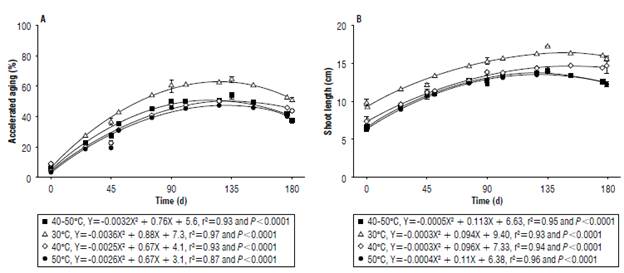
FIGURE 4 A) Accelerated ageing and B) shoot length of black oat seeds, ratures and stored under ambient conditions for 180 days.
Seed vigor, measured by the accelerated ageing test, increased up to approximately 135 d as a result of overcoming dormancy that decreased for all drying temperatures, but with a more pronounced reduction for seeds dried at higher temperatures (Fig. 4A).
Drying temperatures did not cause immediate damage to seed vigor, according to the accelerated ageing test. However, latent drying temperature damage was observed during storage, since seeds subjected to drying temperatures above 30°C showed a lower percentage of normally-germinated seedlings. However, there was immediate damage to the vigor of white oat seeds due to the increase of drying air temperatures, though air temperatures above 50°C were used in that study (Oliveira et al., 2010).
Shoot length showed similar behavior for all drying air temperatures during storage (Fig. 4B). The observed values increased up to approximately 135 d due to overcoming dormancy with a later drop at the end of the storage period. Tunes et al. (2010) and Scariot et al. (2017b) verified the increase in shoot length of barley and wheat seedlings up to 180 and 120 d, respectively, due to the process of overcoming dormancy.
Immediate and latent damage to the shoot length of seedlings has been found due to the increase in the drying air temperature since the seeds subjected to drying at a temperature of 30°C showed the highest values at the beginning and end of the storage period. Similarly, Scariot et al. (2017a) verified immediate damage of the drying temperature increment of up to 50°C on the shoot length of bean seedlings.
Shoot length may be an indicator of seed vigor, since vigor is related to the seed's ability to nourish the embryo, culminating in longer seedlings (Marcos Filho, 2015). Thus, the reduction in shoot length observed with the increase in drying temperature may be related to the thermal damages caused to the embryonic axis or to the reduction of reserves that can be observed in the 1000-seed weight and hectoliter weight of seeds.
The use of the alternating temperature provided better results for germination, germination first count, and germination speed index when compared to drying using only the temperature of 50°C. Electrical conductivity showed better results in relation to the temperatures of 40 and 50°C, when applied individually. However, the results for vigor, measured by the accelerated ageing test and shoot length obtained with alternating temperatures were similar to those obtained when using the highest temperature individually (50°C).
These results demonstrate that the use of high temperatures leads to a reduction in the physiological quality of black oat seeds even when they are alternated with low temperatures, since the analysis of seeds dried at a lower temperature (30°C) showed the best results.
Conclusions
The physical quality of black oat seeds reduces with storage time, regardless of the drying temperature. However, an increase in drying temperature can cause immediate damages to the physical quality of the seeds.
The physiological quality of black oat seeds increases throughout storage until dormancy is overcome, regardless of the drying temperature. However, after dormancy is overcome, the physiological quality of seeds is reduced.
The physiological quality of black oat seeds tends to suffer latently the negative influence of the rise of the drying temperature. Thus, the increase in the drying temperature intensifies the loss of quality throughout storage.
Dry black oat seeds with alternating drying temperatures (40-50°C) showed lower physical and physiological quality than seeds dried at temperatures up to 40°C, but they showed better quality when compared to seeds dried at a temperature of 50°C.














