Introduction
Human population growth has created a demand for crop areas that each year become more productive. However, pests, diseases, and weeds reduce productivity, making agricultural inputs essential for higher yields (Steffen et al., 2011). The application of herbicides is of great importance in world agriculture, as a technology widely used to guarantee high agricultural productivity (Hirakuri & Lazzarotto, 2014).
Glyphosate (N-(phosphonomethyl) glycine) is a post-emergent herbicide that exerts systemic control of a broad spectrum of weeds by inhibiting the enzyme 5-enolpyr-uvylshikimate-3-phosphate synthase (EPSPS) that is responsible for the synthesis of aromatic acids necessary for plant survival (Duke & Powles, 2008; Duke, 2018). Despite the low toxicity of this herbicide, its use can cause contamination of soils and water resources that affects non-target organisms (Haas et al., 2018).
Atrazine (2-chloro-4-ethylamino-6-isopropylamino-s-triazine) is a pre-emergent or early post-emergent and selective herbicide with systemic action (Silva et al., 2017). It is widely used worldwide for weed control in the cultivation of corn, sorghum, and sugarcane (Fan & Song, 2014). Its chemical properties favor the contamination of surface and groundwater due to the high susceptibility of atrazine to leaching and runoff (Fan & Song, 2014).
Due to the fact that chemical control is the most currently used type of control in agriculture, its application has been a cause of concern for society. This is because with the increase in agricultural productivity there is also an awareness of the necessity to maintain environmental quality and human health (Simonato, 2018).
Biological control has acquired importance over the years, contributing to sustainability in the agroecosystem. Its advantages are the low damage to the environment and to human beings, and a greater specificity than chemical pesticides (Oliveira & Ávila, 2010; Wright, 2014).
Some microorganisms, such as those of the genera Azo-spirillum, Bacillus, Saccharopolyspora, and Chromobacterium, have been used in agriculture as growth promoters, biological nitrogen fixators, and biological control agents. These microorganisms have gained importance in the last years, contributing to the sustainability of agroecosystems and reducing damage to the environment and to humans (Palma et al, 2014; Wright, 2014; Caulier et al., 2019).
Despite the importance of herbicides for crop production, the application of these products (including those considered to be of low risk) has negative effects on non-target organisms, such as the microorganisms used in biological control. These effects can be direct, decreasing the abundance of plants, and indirect, impacting microorganisms. Therefore, studies on the harmful effects of pesticides on beneficial organisms are of great importance (Costa et al., 2014; Fonseca et al., 2015; Moscardini et al., 2015; Prosser et al., 2016).
The objective of this study was to analyze the relationship between glyphosate and atrazine applications and the growth of the bacteria Azospirillum brasilense, Bacillus subtilis, Bacillus thuringiensis, Chromobacterium subtsugae and Saccharopolyspora spinosa.
Material and methods
Study location
This research was conducted at the microbiology laboratory of the Federal University of Tocantins, University campus of Gurupi, Brazil (11°43' S, 49°04' N, at an altitude of 280 m a.s.l.). Five different types of bacteria were used: Azospirillum brasilense, Bacillus subtilis, Bacillus thuringiensis, Chromobacterium subtsugae, and Saccharopolyspora spinosa, from the mycological collection of the microbiology laboratory of the University. Each bacterium was evaluated separately to test the effects of the herbicides glyphosate (Roundup Original") and atrazine (Atrazine nortox* 500 SC) on radial growth. These two herbicides are widely used in Brazilian agriculture.
Experimental design
We used a completely randomized design, in a 2x5 factorial arrangement with three replicates per Petri dish, in which factor A corresponded to the two types of herbicides and factor B to the doses of herbicides.
The herbicide doses were calculated according to the manufacturer's recommendation. The reference for glyphosate was a soybean crop and for atrazine a corn crop, using doses ranging from 1.0, 2.0, 3.0, and 4.0 L ha-1 and a control with only distilled water. The concentrations of the active ingredients for the respective doses were 180 g L-1, 360 g L-1, 540 g L-1, and 720 g L-1 of glyphosate, and 250 g L-1, 500 g L-1, 750 g L-1, and 1000 g L-1 of atrazine.
Procedures performed
The herbicide syrups were prepared with distilled and sterilized water with the respective concentrations of the herbicides. The bacteria were multiplied in potato dextrose agar (PDA) culture medium (250.0 g of potatoes, 20.0 g of dextrose, 20.0 g of agar, 250.0 mg of ampicillin to 1.0 L of distilled water) and incubated at 27°C for 7 d.
Subsequently, the bacteria were scratched onto Petri dishes (90 mm) containing the PDA culture medium. The herbicides were added using 10.0 mm diameter filter paper discs. The disks were soaked in the herbicide syrups corresponding to each dose, and then added to the culture medium containing the bacteria. Disks with distilled water were used for the control. After this process, the plates were kept in a biochemical oxygen demand (BOD) chamber (model BT 60 HR, BIOTHEC, Piracicaba, São Paulo, Brazil) under a temperature of 25°C.
The evaluations started 48 h after setting up the treatments, determining radial growth every 48 h, for a total of five evaluations. The measurements were performed with a digital caliper, determining the diameter (mm) of the bacterial growth inhibition halo in three orthogonal directions. If growth was not impeded, the value was equal to zero.
However, with an influence on growth, the total diameter was measured by discounting the value of the paper disk.
Statistical analysis
Data were subjected to an analysis of variance (ANOVA) and the means were compared by the Tukey test (P<0.05). They were then subjected to a multivariate analysis using a principal component analysis (PCA) with the software R* version 3.5.3 (RCore Team, 2013). The graphs were plotted using the software SigmaPlot* version 10.0(SYSTAT, 2014).
Results
According to the ANOVA, herbicides caused changes in bacterial growth (Tab. 1). For the herbicide variable only C. subtsugae showed differences between treatments. For the dose variable, there was a statistical difference for all bacteria. There was an interaction between herbicides and doses, except for B. subtilis.
TABLE 1 Analysis of variance of the diameter of the bacterial growth inhibition halo of the growth of bacteria under the influence of the application of glyphosate and atrazine.

MS - medium square; CV- coefficient of variation; "significant at 1% probability level (P<0.01); "significant at 5% probability level (0.01≤P<0.05); ns - not significant (P>0.05) according to the F test.
According to Figure 1A and B, the bacteria A. brasilense and B. subtilis showed no statistical differences regarding the use of herbicides in the formation of the growth halo. For A. brasilense, the products acted linearly (Fig. 1A), so the highest dose caused a greater inhibition halo (10.40 mm).
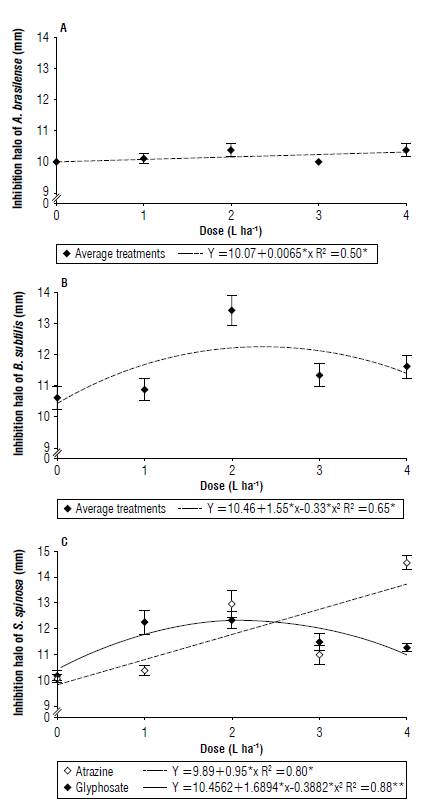
FIGURE 1 Bacterial growth inhibition halo of A) Azospirillum brasilense, B) Bacillus subtilis, and C) Saccharopolyspora spinosa under the effect of the herbicides glyphosate and atrazine (mean ± standard error).
Although the products are indifferent to the formation of halos, B. subtilis showed quadratic behavior (Fig. IB), with the dose of 2 L ha-1 being the most harmful to the bacteria for both products (13.45 mm), 34.5% higher than the control.
Saccharopolyspora spinosa showed a difference for the dose only in the herbicide atrazine. It showed linear behavior (Fig. 1C) with a 12.90 mm halo for the dose of 4 L ha-1 that was 29% higher than the control. Glyphosate showed no statistical difference in terms of doses.
Bacillus thuringiensis and C. subtsugae showed differences in the use of glyphosate or atrazine. Atrazine showed linear behavior, causing a greater inhibition halo with increasing doses. Bacillus thuringiensis showed a halo of 13.66 mm for the dose of 4 L ha-1, representing an 18.8% increase compared to the control. Regarding the same bacteria, glyphosate showed quadratic behavior, with a higher reduction of bacterial growth than atrazine at doses of 1 and 2 L ha-1 (10.78 mm and 15.95 mm, respectively), and an increase of 27.39% compared to the control.
Chromobacterium subtsugae was similarly affected to B. thuringiensis (Fig. 2B). Glyphosate had a greater effect compared to atrazine only at the dose of 1 L ha-1, with a halo of 12.33 mm which corresponds to an inhibition of 7.7% greater than atrazine and 19.7% compared to the control. The same dose of atrazine showed linear behavior and increased the inhibition halo with higher doses. The dose of 4 L ha-1 obtained an inhibition halo of 16.20 mm that was approximately 56% higher than the control.
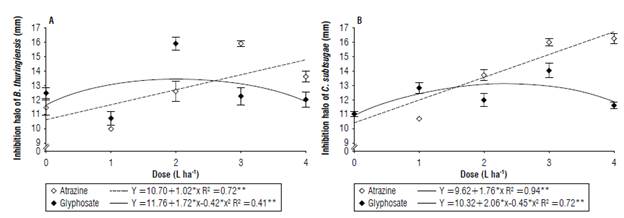
FIGURE 2 Bacterial growth inhibition halo of A) Bacillus thuringiensis and B) Chromobacterium subtsugae under the effect of the herbicides glyphosate and atrazine (mean ± standard error).
According to the principal component analysis (PCA), the data represent a total of 100%. The greater the variance of the component, the greater its degree of importance. The first component (PCI) was responsible for 53.4% of the total variation of the analyzed characteristics regarding the source and doses of herbicides (Tab. 2). According to Hair Jr. et al. (2009), the percentage above 80% of the variance must be approached to determine the adequate number of components. This way, the first three components for the study were selected, which explained 96.6% of the total variance.
TABLE 2 Principal component analysis (PCA), eigenvalues (λi), and percentage of explained variance and cumulative variance (%) by components.

PCI (53.4%) best represented the relationship between herbicide responses and doses and inhibition of bacterial growth, positively associated with S. spinosa (0.87), A. brasilense (0.77), C. subtsugae (0.68), B. subtilis (0.67), and B. thuringiensis (0.63). For the second component (PC2) (22.4%), the highest coefficients were C. subtsugae (0.64), B. thuringiensis (0.44), and S. spinosa (0.00), with B. subtilis (-0.44) and A. brasilense (-0.55) showing a negative correlation.
Regarding the third component (PC3), B. thuringiensis (0.61) and B. subtilis (0.57) showed the highest positive variance coefficients. In contrast, S. spinosa (-0.44), A. brasilense (-0.28) and C. subtsugae (-0.26) showed a negative correlation (Fig. 3).

FIGURE 3 Coefficient of variation of variables correlated with the three main components A) PC1, B) PC2, and C) PC3 in bacteria Saccharopolyspora spinosa (SS), Azospirillum brasilense (AB), Bacillus subtilis (BS), Bacillus thuringiensis (BT), and Chromobacterium subtsugae (CS).
Regarding the effects of treatments on the three main components (Tab. 3), for PCI the treatments that showed the highest influence were atrazine 4 L ha-1 (2.14), atrazine 2 L ha-1 (1.99), and glyphosate 2 L ha-1 (1.65), and the lowest responses were observed in treatments atrazine 1 L ha-1 (-2.19), atrazine 0 L ha-1 (-2.06), and glyphosate 0 L ha-1 (-1.66). In PC2, the highest values were found in the treatment atrazine 3 L ha-1 (1.99) and the lowest in glyphosate 4 L ha-1 (-1.34) and atrazine 2 L ha-1 (-1.23). For PC3, the treatments with the lowest values were glyphosate 2 L ha-1 (1.64), atrazine 3 L ha-1 (1.12), and atrazine 4 L ha-1 (-1.66).
The PCA results were plotted on a biplot chart (Fig. 4). The doses of atrazine 2 L, 3 L, and 4 L ha-1 and glyphosate 2 L ha-1 were responsible for the largest growth inhibition halos. We also observed that doses below 1 L ha-1 of atrazine and glyphosate obtained the best responses, thus significantly influencing bacterial growth.

FIGURE 4 PC1 x PC2 biplot of the variable responses of doses and sources of herbicides for bacterial growth inhibition. Bacillus thuringiensis (BT), Bacillus subtilis (BS), Azospirillum brasilense (AB), Chromobacterium subtsugae (CS) and Saccharopolyspora spinosa (SS).
Chromobacterium subtsugae and B. thuringiensis were more influenced by the atrazine treatments at doses 3 L and 4 L ha-1, while the A. brasilense, B. subtilis, and S. spinosa were more inhibited in terms of their growth at the doses of 2 L ha-1 atrazine and 2 L ha-1 glyphosate. It is noteworthy that when using lower dosages than these, the inhibition of microbial growth became lower, thus not affecting the final development of the bacteria.
Discussion
Changes in bacterial growth were observed. The linear effect of atrazine doses on A. brasilense, S. spinosa, B. thuringiensis, and C. subtsugae is related to the mechanism of action of the herbicide and tolerance of bacteria to the product. Atrazine causes membrane rupture, dehydration, and disintegration of cells and organelles through the oxidation of lipids and proteins (Oliveira Jr., 2011).
Inhibition of bacterial growth due to the use of atrazine may be related to a lower absorption of nutrients present in the culture medium, causing stress to the bacteria. Thus, part of the energy available for the development of bacteria is lost to the maintenance of cellular and biochemical mechanisms, affecting their growth (Schimel et al, 2007).
Bacillus subtilis can metabolize very high concentrations of atrazine. In some cases, there is a description of urea formation from biuret or allophanate that can be cleaved by the urease enzyme releasing CO2 and 2NH3, besides the rapid degradation of cyanuric acid which can serve as nitrogen source for bacteria (Wang et al., 2014).
Glyphosate application affected the growth of bacteria. In A. brasilense, it followed the same behavior of atrazine, increasing the effect on the inhibition halo with higher herbicide doses. This showed that the bacterium did not show tolerance to either of the two molecules.
Among the tested doses of glyphosate, the most harmful was 2 L ha-1 (Fig. 4), causing a greater inhibition halo, an effect linked to the ability of glyphosate to acidify the medium, decrease cell density, and provide unfavorable conditions for bacterial growth (Manogaran et al., 2017).
The reduction of bacterial growth at doses above 2 L ha-1 is directly linked to the composition of glyphosate. This herbicide is an organophosphate consisting of carbon-phosphorus bonds that allow its easy degradation by a select group of microorganisms that use phosphorus from glyphosate degradation for their development. Additionally, other bacteria have the ability to adapt to the stress that the herbicide can cause, not compromising their development.
The primary and predominant metabolites of microbial degradation in glyphosate are glyoxylate and aminomethyl phosphonic acid (AMPA) that turn into water, carbon dioxide, and phosphate (Carranza et al., 2019). The AMPA metabolite can later be transformed into phosphate and methylamine by the action of a C-P lyase and/or into phosphate and formaldehyde by the combined action of a transaminase and a phosphonatase (Carranza et al., 2019; Artigas et al., 2020). Unlike the AMPA pathway, some bacteria such as Bacillus sp., Pseudomonas sp. and others, can metabolize glyphosate into sarcosine using this component as a growth nutrient (Fan et al, 2012).
The ability of bacteria to use glyphosate as a source of phosphorus for the synthesis of their cellular components is determined by the presence of a C-P lyase enzyme system that breaks the C-P bond to form non-toxic components, like sarcosine (N-methylglycine) and orthophosphate (Kryuch-kova et al., 2014). However, the uptake of glyphosate by bacterial cells and its subsequent degradation by the C-P-lyase pathway are induced only when other sources of P are scarce (Fitzgibbon & Braymer, 1988).
Bacteria that can use glyphosate as a source of phosphorus are associated with adaptation directed by a genetic mutation in which the isolate uses the herbicide for its cell propagation (Dibua et al., 2015). This result was similar to that found in the present study in which the bacteria B. subtilis, B. thuringiensis and C. subtsugae showed a lower interference of the herbicide in the growth of bacteria at doses of 3 L ha-1 and 4 L ha-1 compared to the lowest doses of1 L ha-1 and 2 L ha-1. This suggests that the bacteria used the herbicide as the only source of phosphorus present in the culture medium for its growth.
Regardless of the glyphosate concentration, this herbicide can cause harmful effects on B. thuringiensis, causing a detrimental effect on its development and formation of colonies (Agostini et al, 2013).
Conclusions
The herbicides glyphosate and atrazine affect the development of the studied bacteria. However, atrazine has an increasing relationship between doses and inhibition of bacterial growth. Regardless of the herbicide, the higher the dose, the greater the growth inhibition halo for bacteria B. thuringiensis, C. subtsugae, S. spinosa, and A. brasilense. The bacterium B. subtilis can degrade high doses of atrazine in the medium, demonstrated by the smaller halo of bacterial growth.
In general, the glyphosate dose that most affected the development of bacteria was 2 L ha-1; higher doses affected the development of bacteria less. According to the package leaflet of the herbicide, the most frequently recommended doses range from 0.5 to 2 L ha-1, coinciding with the most harmful dose of the product in the present study. Thus, the data presented in this paper provide relevant information regarding the use of bacteria in agriculture and the effects that agricultural pesticides may cause in their development, which may help to support decisions made by growers.















