Introduction
Bone marrow is responsible for hematopoietic production. However, in some cases, it could be invaded by non-hematopoietic cells secondary to the spread of a solid tumor1. This affair could lead to cytopenias and leukoerythroblastic reactions, defined as anisopoikilocytosis, immature cells, and abnormal erythroid shapes in the peripheral blood smear2.
It should be noted that bone marrow involvement in hematologic malignancies is relatively more common and clearly described in the literature3. Furthermore, some diseases primarily originate in the bone marrow, such as leukemia and multiple myeloma; using the term metastasis is imprecise in these diseases. In fact, for malignant hematologic disorders, clinical practice guidelines provide specific recommendations on when to perform histopathological studies of the bone marrow as part of staging or even to use surrogate exams such as PET CT or MRI, which could, in some cases, replace the need for biopsy or aspiration4. Conversely, in solid tumors, the data is less clear and heterogeneous across different reports, and there is no consensus on the approach to possible marrow involvement for all neoplasms. In addition, some data is heterogeneous, presenting a prevalence of less than 10-40 % of marrow involvement in carcinomas, but in other studies, up to 70 % of bone metastases have been found in patients who die from breast or prostate cancer5-7.
Recently, bone marrow invasion or infiltration by non-hematological malignant cells has been gaining scientific interest8. However, there needs to be more agreement on some descriptive aspects, such as the modality of diagnosis, that could impact the reported distribution of the primary source of bone marrow metastasis9. Furthermore, the available information on this topic is found in retrospective cohorts or case-control studies with diverse incidences for each of the different neoplasms, so there needs to be condensed data to compare the information between various reports and strategies. That is why a systematic review of the literature was performed to offer a global, comprehensive, and descriptive view of this condition, with the primary objective of recognizing the principal sources of bone marrow metastasis in adults by non-hematological tumors confirmed by histopathological studies. Other essential variables such as a diagnostic modality, laboratory findings such as cytopenia, leukoerythroblastic reaction, and survival are also discussed, if available.
Methods
Data source and search Strategy
Using the PRISMA 2020 recommendations in a prespecified protocol, the researchers conducted a systematic review of published studies on PubMed/ Medline (primary database) and Google Scholar (secondary citation searching source) related to the standardized search terms: "Myelophthisis", "Panmyelophthisis", "leukoerythroblastic anemia" (MeSH), "Myelophthisic anemia" (MeSH), "Malignant leukoerythroblastic" and "Bone marrow metastasis" (MeSH). We manually crossed non-specific terms "neoplasm", "cancer", and "malignancy". In this phase, no terms were used to restrict the search to non-hematologic diseases because the selected keywords are theoretically limited to solid tumors. Words such as 'metastasis to the bone marrow by lymphoma or myeloma' are inappropriate. Conversely, 'metastasis to the bone marrow from non-hematologic tumors' is redundant. In practice, we selected a strategy with higher sensitivity in the initial search and subsequently refined the selection through an exhaustive review of candidate studies for definitive inclusion because of the origin of the malignancy.
Inclusion criteria were reports with the availability of histopathological confirmation of bone marrow invasion by malignant non-hematolymphoid cells reported by oncology or pathology centers. We excluded literature secondary information and case report series with methods restricted to a specific neoplasm trying to reduce selection bias. Eligible studies could be multicentric and had no restriction because of the cancer subtype. We do not consider the indication or specific method of the bone marrow studies as an exclusion criterion either. The selection was further refined by filtering the papers by human studies, adult age (>14 years old), and thirty years between 1990 and May 2021 that correspond with the implementation of the NCCN and WHO classification of tumor guidelines10,11. Before these classification consensuses, the reports included nosological terms that could be inappropriate or non-homologous. We omitted conferences, comments, editorials, letters, lectures, audio-visual material, topic reviews, and study subtypes not intended to provide clinical information about patients with the interest condition. The main goal was to establish and compare the distribution of reported primary sources of bone marrow metastasis. Those studies with enough information to identify subgroups let us exclude cases with confusing reports that could be considered a significant source of bias. For example, we excluded children's patients or protocols with arbitrary differences in the diagnostic approach of cases categorized as unknown primary origin neoplasm to avoid a "lack of immunohistochemistry bias". This approach was adopted to forestall potential biases in the comparison and results of the current review.
After eliminating duplicates and non-complete available text issues, we evaluated full preselected text articles. We did a second review to check the prespecified inclusion criteria in every item. A third person solved discrepancies between reviewers if a consensus still needed to be reached.
Data extraction, synthesis, and analysis
Because of the high clinical and methodological heterogeneity, pooled data was not metanalysed, but a qualitative synthesis analysis was performed. The relevant variables, such as age, gender, cancer diagnosis, bone marrow test method, peripheral blood abnormalities, and mortality, were extracted for two searchers independently and recorded in an electronic preform template; this process was crosschecked again for a third independent researcher. Finally, data were summarized and presented in tables in a descriptive format.
Lastly, a quality assessment of the observational studies was conducted utilizing the STROBE checklist to evaluate, compare, and describe the reporting process of most included items. We classified high, medium, or low adherence if they complied with more than 19, between 16-19, or under 16 of the STROBE recommendations.
Results
We identified 8911 records principally from MedLine (Figure 1). After excluding duplicates, not retrieved papers, and automatically filtered issues with Rayyan® software, we got 62 articles for full-text revision. Finally, we selected 31 papers for inclusion. One of them (Chou et al.) presented two different series, so we preferred to describe each apart for a total of 32 patient cohorts.
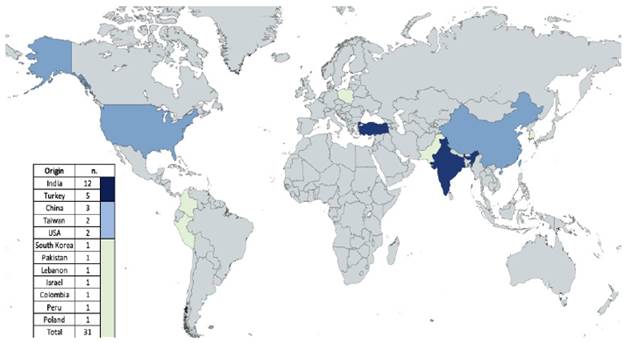
The 31 included papers come from 12 countries (Figure 2); four were multicentric, and all but 2 were retrospective. We report pool data of 1451 adult patients with solid tumors bone marrow metastasis with a mean age of 53 years (14-91 years) and male sex in 63 % of cases. Both characteristics were calculated with 23 and 24 of the included studies, respectively. Twenty-four pathology centers screened 83277 bone marrows by smear, biopsy, imprint, or combined methods, finding 837 BMM in the inclusion period.
All the included studies presented the distribution of the primary source of metastasis. Those reports are combined in Table 1, ranked by sample size and cancer subtype. Eighty-two percent of cases were epithelial neoplasms (n=927), 14 % (n=211) were tumors of unknown primary origin, and 10 % (n=146) were low-frequency specific neoplasm grouped as "others" in a miscellaneous category shown at the bottom of the same table. Because of insufficient histopathology data, we could not use a deeper classification strategy other than the anatomic origin of neoplasm, with some exceptions.
Table 1 Distribution of primary source of metastasis ranked by specific neoplasm, study, and sample size.
| First Author, year (ref) | Origin | Period (Months) | Design | 1. Breast | 2. Lung | 3. Prostate | 4. Unknow Primary | 5. Stomach | 6. Others* | 7. Pharynx | 8. Colorectal | 9. PNET / Ewing | 10. Kidney and UT | 11. Muscle | Total of patients |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Chou et al. 201512 | Taiwan | 48 | M/R | 37 | 21 | 19 | 0 | 43 | 0 | 8 | 10 | 0 | 0 | 0 | 138 |
| 24 | 25 | 23 | 18 | 21 | 0 | 19 | 6 | 0 | 0 | 0 | 136 | ||||
| Luján et al. 200913 | Colombia | 188 | U/R | 27 | 4 | 9 | 19 | 7 | 9 | 0 | 0 | 2 | 3 | 9 | 89 |
| Hung et al. 201414 | Taiwan | 156 | U/R | 9 | 12 | 16 | 7 | 32 | 0 | 0 | 7 | 0 | 0 | 0 | 83 |
| Rani et al. 202115 | India | 216 | U/A | 20 | 12 | 12 | 25 | 0 | 10 | 0 | 0 | 0 | 3 | 0 | 82 |
| Kiliçkap et al. 200716 | Turkey | 144 | U/R | 21 | 17 | 5 | 6 | 7 | 7 | 3 | 1 | 5 | 1 | 0 | 73 |
| Filanovsky et al. 201717 | Israel | 129 | U/R | 21 | 16 | 16 | 9 | 0 | 10 | 0 | 0 | 0 | 0 | 0 | 72 |
| Krishan et al. 200718 | USA | 216 | M/R | 31 | 9 | 6 | 3 | 2 | 10 | 0 | 0 | 1 | 3 | 0 | 65 |
| Aksoy et al. 200719 | Turkey | NR | U/R | 14 | 14 | 0 | 8 | 9 | 16 | 0 | 0 | 0 | 0 | 0 | 61 |
| Xiao et al. 200820 | China | 144 | M/R | 9 | 11 | 5 | 9 | 11 | 8 | 3 | 1 | 0 | 2 | 0 | 59 |
| Kucukzeybek et al. 201421 | Turkey | 91 | U/R | 23 | 3 | 4 | 19 | 6 | 0 | 1 | 1 | 0 | 1 | 0 | 58 |
| Moid et al. 200522 | USA | 130 | U/R | 21 | 11 | 9 | 3 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 46 |
| Brahmbhatt et al. 201423 | India | 42 | U/R | 4 | 4 | 3 | 9 | 1 | 6 | 1 | 1 | 11 | 0 | 4 | 44 |
| Loayza et al. 199924 | Peru | 139 | U/R | 0 | 3 | 25 | 3 | 3 | 2 | 0 | 0 | 0 | 1 | 0 | 37 |
| Yun et al. 200725 | S. Korea | 138 | M/R | 1 | 5 | 2 | 9 | 17 | 2 | 1 | 0 | 0 | 0 | 0 | 37 |
| Gahlot et al. 202026 | India | 67 | U/R | 4 | 6 | 6 | 5 | 0 | 6 | 2 | 2 | 0 | 3 | 0 | 34 |
| Mehdi et al. 201127 | India | NR | U/R | 8 | 4 | 9 | 1 | 5 | 2 | 0 | 0 | 1 | 1 | 0 | 31 |
| Zhou et al. 201828 | China | 72 | U/R | 5 | 3 | 3 | 5 | 7 | 6 | 1 | 0 | 0 | 0 | 0 | 30 |
| Aytan et al. 201929 | Turkey | 48 | U/R | 11 | 4 | 4 | 2 | 4 | 5 | 0 | 0 | 0 | 0 | 0 | 30 |
| Farah et al. 201830 | India | 36 | U/R | 7 | 9 | 3 | 2 | 1 | 5 | 0 | 0 | 0 | 0 | 0 | 27 |
| Rudresha et al. 201931 | India | 36 | U/R | 3 | 0 | 2 | 0 | 1 | 8 | 2 | 2 | 5 | 1 | 2 | 26 |
| Wong et al.199332 | China | 93 | U/R | 0 | 13 | 3 | 5 | 1 | 1 | 0 | 2 | 0 | 0 | 0 | 25 |
| Chandra et al. 201033 | India | 44 | U/R | 1 | 4 | 10 | 4 | 2 | 3 | 0 | 0 | 1 | 0 | 0 | 25 |
| Naveen et al. 200734 | Pakistan | 33 | U/R | 3 | 7 | 5 | 9 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 24 |
| Tyagi et al. 201735 | India | 36 | U/R | 3 | 0 | 3 | 10 | 1 | 3 | 0 | 0 | 1 | 1 | 0 | 22 |
| Ozkalemkas et al. 200536 | Turkey | 108 | U/R | 0 | 2 | 3 | 5 | 8 | 0 | 0 | 0 | 0 | 0 | 1 | 19 |
| Kumar et al. 201937 | India | 60 | U/R | 1 | 0 | 8 | 7 | 0 | 1 | 0 | 2 | 0 | 0 | 0 | 19 |
| Chauhan et al. 201638 | India | 40 | U/R | 3 | 2 | 2 | 2 | 0 | 2 | 0 | 0 | 1 | 1 | 2 | 15 |
| Mishra et al. 201439 | India | 60 | U/R | 1 | 2 | 0 | 1 | 1 | 2 | 0 | 3 | 2 | 1 | 0 | 13 |
| Bashir et al. 201840 | India | 35 | U/R | 3 | 4 | 2 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 11 |
| Hamid et al. 200941 | Lebanon | 11 | U/P | 3 | 2 | 0 | 1 | 2 | 1 | 1 | 0 | 0 | 0 | 0 | 10 |
| Kolda et al. 201742 | Poland | NR | U/R | 1 | 0 | 2 | 4 | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 10 |
| Total of patients | 319 | 229 | 219 | 211 | 193 | 128 | 42 | 38 | 31 | 23 | 18 | 1451 | |||
*Others (n.128): Non-specified (n.20), Neuroblastoma (n.11), Ovaric (n.11), Neuroendocrine (n.9), GIST (n.8), Gastrointestinal (n.7), Melanoma (n.7), Biliar (n.6), Soft Tissues (n.6), Cervix (n.6), Liver (n.4), Osteosarcoma (n.4), Head & Neck (n.4), Thyroid (n.3), Skin (non-melonoma)(n.3), Germ Cell (n.2), Parathyroid (n.2), Endometrium (n.2), Pancreas (n.2), Thymus (n.2), Sweat Gland (n.2), Esophagus (n.2), Choriocarcinoma (n.1), Testes (n.1), Adrenal Gland (n.1), Meninges (n.1), Angiosarcoma (n.1), Salivary Gland (n.1).
Source: authors
In Table 2, hematologic variables reported in the studies are presented when available. Anemia, thrombocytopenia, and the leukoerythroblastic reaction were the most frequent manifestations in peripheral blood. Moreover, more than half of the cases showed some fibrosis in histology.
Table 2 Hematologic variables
Source: authors
Ten studies with 775 patients reported a non-tumor-specific 2.5 months survival from bone marrow metastasis diagnosis. No other outcome variables were presented consistently between studies to complement these results.
Finally, we proposed the STROBE checklist strategy to evaluate the communication quality of the papers included in this review. All by one were observational cross-sectional studies; seven were classified as high and twelve as medium and low STROBE adherence issues, respectively.
Discussion
Multiple terms are used in clinical practice to refer to non-hematopoietic bone marrow cancer infiltration. However, medical expressions like "Myelophthisis" or "Myelophthisic anemia" should not be used because of confusion. On the other hand, Bone Marrow Metastasis (BMM) is the most common and generally recognized term to communicate this entity in literature. It offers more clarity and consistency with the growing idea of considering the medullary compromise by solid tumors as early-stage bone metastasis42,43. If so, we should expect a higher reported incidence of this condition, but it is still considered a "rare" entity in most available references.
This underestimation occurs because of a nihilistic perspective of the oncologic patient with metastatic disease. It produces a "systematic exclusion" of patients for bone marrow studies, adducing futility and reducing the apparent prevalence of this condition. Another source of underestimation is the geographically restricted report of non-compared and small sample-sized cohorts that do not represent the actual cancer epidemiology and cannot be generalized. That's why we consider it essential to present the comparison of the most significant international case series (table 1) to give the reader a general idea of the available data on bone marrow metastasis.
The most frequently reported tumors in the BMM adult cohorts are epithelial neoplasms such as breast, prostate, lung, or stomach cancer, representing more than 70 % of the primary source of infiltration in this review. This finding is expected because epithelial tumors are the most common malignancies worldwide44. These common primary tumors have similar characteristics in the peripheral blood findings, like the leukoerythroblastic reaction, which can occur occasionally in hematologic neoplasms but is observed most frequently in some solid tumors such as the prostate, breast, or lung tumors. At the same time, we can find some particularities between them. For example, breast and prostatic carcinoma are associated with myelofibrosis, which sometimes has been reversible with successful therapy for the diseases; or in small cell lung carcinoma, when marrow compromise is found, usually other metastasis sites are associated44. Also, we have significant differences in survival rates between those tumors after metastasis, as we can see in a Danish cohort study in 2017, where breast carcinoma has the best survival rates and lung carcinoma the lowest ones (51 % vs. 10 % at one year, 25 % vs. 2 % at three years and 13 % vs. 1 % at five years).
It is also recognized that epithelial neoplasms predominate in adults and mesenchymal neoplasms in younger individuals. Interestingly, we found Ewing's sarcoma among the top ten of neoplasias, even in "adults" (>14 years). However, most of the studies included did not provide age-specific data for each tumor subtype. So, it was impossible to analyze distribution by age groups to define whether patients with Ewing's sarcoma/PNET occurred in the youngest fraction of the cases. As reported before, we would expect these cases to predominate in adolescents and young adults11.
Another exciting aspect is that pharyngeal carcinomas represented a significant group in the rank list, coming mainly from Taiwan, China, and Turkey, which are areas of a relatively high incidence of this type of neoplasia compared to cohorts from other places12,16,19,20,28,32,36. The same geographic differential behavior occurs with gastric cancer, which is more prevalent in Taiwan, China, and South Korea cohorts. They all reported this neoplasm as their leading cause of bone marrow metastasis25. The general prevalence of those tumors likely depends on the frequency of exposure to some known cancer risk factor restricted to specific geographic areas, affecting the incidence of the primary source of BMM as well. As expected, the distribution of neoplasms also depended on the sex proportion of cases in each cohort. Those papers that did not register breast cancer tended to contain mainly male patients even though they did not report any per protocol restriction or selection criteria to explain this sex predominance. We could explain these differences in the reported rankings because of center specialization, sociocultural, or health system bias.
Almost a fifth of the cases were neoplasms of unknown primary origin. Still, most of the studies did not report on immunohistochemical protocols, which could explain the significant variability of this subset of patients among the different included papers (3-50 %). On the other hand, the considerable diversity of low-frequency neoplasms grouped in "other" is remarkable. It shows that virtually, any neoplasm can compromise the bone marrow via the hematogenous route45,46.
There is a theoretical concern about difficulty detecting malignant cells because infiltration can be focal and induce different fibrotic changes in the BM20. In addition, some malignant epithelial cells have intercellular cohesive unions, causing neoplastic cell adhesion and interfering with its recovery by aspiration. In this review, fibrosis/desmoplasia occurs in 57 % of cases, and up to a quarter of aspirates got a dry tap. It was similar to a study that analyzed the "dry tap etiology," reporting malignant disease as the most common cause of this finding47. Unfortunately, we could not pool data on BMM diagnostic modalities. Nevertheless, most studies with available data reported a better detection rate for biopsy than aspirate. Only two evaluated bone imprinting as a complementary strategy, and none had "new techniques", such as flow cytometry or Polymerase Chain Reaction PCR, that are very useful and could complement the evaluation because of better sensitivity in some circumstances48-50.
Nonetheless, these techniques are only generally available, affordable, and validated in a few pathology centers. Until now, a complete approach using morphology with standard stains or immunochemistry panels is the best option13,17,29. In general, the recommendation is to routinely complement the diagnostic process by combining the biopsy, aspiration, and imprinting simultaneously to improve the probability of metastasis detection.
Most patients had peripheral blood alterations in one or more lines. The most frequent was anemia, but the most specific was the leukoerythroblastic reaction reported in almost 50 % of the patients in this study. A recently published systematic review says that approximately 63 % of the causes of the leucoerythroblastic picture are due to underlying malignancy, the majority (40 %) due to solid tumors51. Another substantial alteration is thrombocytopenia because several studies have identified it as a prognostic marker in multivariate analyses14,19,25. Indeed, in the Luján et al. cohort, one of the leading causes of death was severe bleeding in the central nervous system and digestive tract13. It would be ideal that future investigations will account for the hematological alterations since not all the articles included in this review present this data.
This review did not consider studies implementing new diagnostic techniques, such as the diagnosis approaches of marrow infiltration through advanced imaging methods, because those are neither universally available nor comparable with clinically based selection and performance of pathology tests in bone marrow metastasis for all different tumors. Nevertheless, it is recognized that with the availability and integration of imaging studies with a significant capacity for bone marrow assessment, such as magnetic resonance imaging or nuclear medicine methods (PET), changes in the reported data in clinically and histopathologically based studies are expected. Shortly, this test will identify early infiltration of the BM niche before clinical or macroscopic structural compromise appears, leading to early interventions. There is evidence that treatment at that moment prevents the establishment of frank bone metastasis and its complications52-54, changing the prognosis dramatically.
The average survival after the diagnosis of bone marrow metastases is only 2.5 months. Like other variables of this clinical entity, selection biases will likely influence it. BMM prognosis depends mainly on the primary tumor and the evolution of the oncologic disease. Some studies have identified other predictive variables like the number of organs affected by metastasis, visceral disease, hemoglobin, LDH, alkaline phosphatase, performance status, and platelet count. Chou et al. presented the most extensive sample size study of BMM12 with a validated model called the "marrow metastases prognostic score (MMPS)."
The descriptive nature of the included studies, the lack of robust methodology data, or detailed definition of the selection criteria in most of them increase the risk of confusion and bias. It is important to emphasize that the collected information cannot be interpreted as the actual prevalence of bone marrow metastasis in cancer patients, nor can it define the specific probability of bone marrow infiltration for each tumor subtype. Indeed, prevalence data is likely underestimated in these retrospective studies. In practice, many patients with clinical or subclinical bone marrow involvement by cancer are not subjected to biopsy, aspiration, or marrow imprint studies, and they will never be reported, especially if they can not access experienced oncology centers. Conversely, the performance of biopsy, aspiration, and imprint may be overestimated in some studies because participating patients are preselected and have a higher pretest probability, mainly if the examinations were prompted by blood abnormalities or other evidence of possible marrow metastasis. We tried to reduce selection bias, excluding a small sample size (less than ten) series of tumor-specific protocols with arbitrary inclusion criteria. However, It was not possible to exclude the possibility of increasing the risk of bias of rejecting "not-so-prevalent tumors that are potentially metastatic or frequent tumors that are rarely metastatic" using this strategy.
To overcome these methodological problems, we suggest a prospective, ideally multicentric study with a significant and heterogeneous sample size that does not restrict participation by neoplasm subtype, symptoms, hematologic abnormality, performance status, or cancer staging. In such a study, criteria and time points for bone marrow evaluation with standardized methods should be specified a priori. Despite the described limitations, this is the first effort to compile and present histopathological data reports for bone marrow infiltration by solid tumors. We recognize that the results presented are just a proxy of the actual behavior of this entity. The submitted data must be interpreted with caution.
Conclusion
The pooled data in this review suggest that the primary sources of bone marrow metastasis in adults are epithelial tumors. Nonetheless, other cancer subtypes are not depreciable, and almost any malignancy can have hematologic spread to bone marrow. In addition, polled patients presented frequent, "easy-to-evaluate'' peripheral blood alterations, including leukoerythroblastic picture. It is recommended that clinicians be aware of this condition and its prognostic meaning.
Combining diagnostic resources to identify BMM in cancer patients increases the empiric possibility of detection. Further research should investigate whether earlier detection of medullary involvement by cancer can translate into valuable interventions that may impact patient clinical outcomes. The reader should evaluate these results with attention due to the significant heterogeneity and high risk of bias.