Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista de la Universidad Industrial de Santander. Salud
Print version ISSN 0121-0807On-line version ISSN 2145-8464
Rev. Univ. Ind. Santander. Salud vol.42 no.2 Bucaramanga May/Aug. 2010
in the treatment of oral cancer
Leandro Santos Bicalho1, João Paulo Figueiró Longo1, Inés Villamizar1,
Maria de Fátima Meneses de Almeida Santos1, Ricardo Bentes Azevedo1.
1. BDS, Department of Genetics and Morphology, University of Brasilia.
Correspondence: Ricardo Bentes Azevedo, Universidade de Brasília, Instituto de Ciências Biológicas, Departamento de Genética e Morfologia,
Brasília - DF. Zip Code: 71910-900. Phone: 55.61.31073081. Fax: 55.61.31073081. E-mail: razevedo@unb.br
Received: 10 May 2010 - Accepted: 25 July 2010
Terapia Fotodinámica, un nuevo acercamiento
en el tratamiento del cáncer oral
RESUMÉN
La incidencia del cáncer de cabeza y cuello es aproximadamente 640.000 casos nuevos por año. El cáncer oral representa un tercio de todos los cánceres y es el octavo más diagnosticado en hombres. Los tratamientos más usados para estos tumores son la cirugía, radioterapia, quimioterapia, o una combinación de estas modalidades terapéuticas. Debido al gran deterioro estructural, funcional y estético que causan los tratamientos convencionales, muchos estudios buscan nuevos métodos para remplazar o asistir el tratamiento del cáncer oral. La terapia fotodinámica (PDT) es una nueva modalidad promisoria en el tratamiento del cáncer que apenas está siendo usada clínicamente. Este artículo describe el uso de PDT como una alternativa para el tratamiento del cáncer oral. Salud UIS 2010; 42: 167-174
Palabras Claves: Terapia fotodinámica, cáncer oral, tratamiento
ABSTRACT
The worldwide incidence of head and neck cancer is, approximately, 640,000 new cases per year. Oral cancer accounts for one third of all cancers and it is the eighth most diagnosed in men. The most used treatments for these tumors are surgery, radiotherapy, chemotherapy, or a combination of these therapeutic modalities. Due to the large structural, functional and aesthetic impairment that conventional treatments cause, many studies seek new methods to replace or to assist the treatment of oral cancer. Photodynamic therapy (PDT) is a promising new modality of cancer treatment that is already being used clinically. This article describes the use of PDT as an alternative for the treatment of oral cancer. Salud UIS 2010; 42: 167-174.
Keywords: Photodynamic therapy, oral cancer, treatment
CANCER
The harmonization of several processes involved in the proliferation and differentiation of cells is indispensable for the development and maintenance of the organism. The disruption of this integration can result in a number of diseases, including cancer, a generic term that covers more than 200 kinds of diseases. Currently, cancer has become one of the most important public health problems worldwide. Epidemiological data indicate that malignancies are the third leading cause of death in the world and the second among the diseases1.
According to the report of the International Agency for Research on Cancer (IARC) / WHO (World Cancer Report, 2008)2, the overall impact of cancer more than doubled in 30 years. Currently, cancer is one of the most common causes of morbidity and mortality worldwide. It has been reported over 10 million new cases and over 6 million deaths per year. Over 20 million people worldwide are living with a diagnosis of cancer and more than half of all cancer cases occur in developing countries. The continued population growth, as well as their aging, affect significantly the impact of cancer in the world. This impact will primarily have an effect on the countries of medium and low development levels. The IARC / WHO estimated that half of new cases and nearly two thirds of cancer deaths occur in these locations3.
Cancer is a terminology known worldwide for a variety of diseases that have in common the proliferation of specific cell populations, with potential to invade nearby and/or distant tissues. In pathological terms, cancer is classified as a malignant neoplasm. In other words, it is the development of new cell clones with greater proliferative activity than neighboring cells and with severe invasive and destructive potential4.
Normal cells proliferate as a result of a biochemical balance between growth factors and inhibitors. These factors reach the cells through the circulation and from neighboring cells. Cancer cells are not affected by these regulatory mechanisms, following its own internal control for replication. Thus, their growth is unrestricted and these cells can escape from senescence and cell death programs5.
Some authors describe the development of cancer as a microevolutionary process at cellular scale, where one of the billions of cells in the human body develops a new feature that allows a higher proliferative activity despite the inhibitory signaling of the body. The number of cells within tissues is strongly regulated by both intracellular mechanisms, such as the induction of apoptosis and DNA repair, and by extracellular control, such as tumor suppression by the immune system. The occurrence of disordered proliferation, as in the case of cancer, occurs through a failure in the regulation of these mechanisms or through the development of malignant clones that escape from them6.
In general, the carcinogenic process is slow and may even be divided into three phases: initiation, promotion and progression. In the initiation phase, the cells are exposed to carcinogens that may promote cumulative changes or genetic instability. The promotion stage is characterized by the proliferation and selection of clones affected in the first phase. These cells start the process of malignant transformation characterized by the expression of oncogenes. At the stage of disease progression, preneoplastic cells develop into malignant neoplastic cells through a process of clonal expansion, which is facilitated by the progressive increase in genetic instability in these cells7.
ORAL CANCER
Oral cancer refers to a subgroup of head and neck tumors that develop on the lips, tongue, salivary glands, gums, floor of mouth, hard palate, buccal mucosa and other intra-oral locations. These cancerous lesions are among the most prevalent forms of cancer in the world population8.
Oral cancer is the most common cancer among head and neck tumors, accounting for about one-third of all such tumors. According to current estimates, about 275,000 new cases of oral cancer are diagnosed each year in the world9, 10. In addition to this high incidence, approximately 130,000 deaths per year are caused by this disease. The incidence of oral cancer in Latin America is approximately 4.6% of the population. However, most of the data for these statistics come from studies on Brazil, which complicates the description of the patterns of incidence in this continent11.
Oral cancer ranks eighth among the highest incidence of cancers in men worldwide2. Brazilian male population has the third biggest risk for the development of oral cancer, second only to France and India10. According to estimates by the National Cancer Institute (INCA)1, in Brazil, oral cancer is the fifth most frequent cancer among men and the sixth among women. In addition, more than 10,000 new cases of mouth cancer are diagnosed each year in this country.
It is believed that the differences in incidence of tumors in the oral cavity in distinct populations are directly related to the different degrees of exposure to risk factors in these groups. In most countries around the world, oral cancer is more common in men than in women. The higher prevalence in men compared to women can be explained by their greater exposure to risk factors for oral cancer, such as tobacco, alcohol, and ultraviolet radiation from sunlight12. The proportion of men to women diagnosed with oral cancer; however, has diminished in recent decades and now it is about 1.5:113.
Risk factors for developing oral cancer are well established in the literature. The excessive consumption of alcohol and tobacco is considered the main etiological factor for the development of tumors in the mucosa and the exposure to solar ultraviolet radiation for lip cancers. Other factors such as diet composition, viral infections and hereditary factors are also considered potential risk factors9, 14-18.
In the oral cavity, approximately 90% of malignant lesions are carcinomas, which are tumors of epithelial origin. They can be identified and treated more easily in their early stages of development. Early diagnosis of these tumors can be accomplished with relative ease due to the accessibility of the oral cavity for examination. However, the vast majority of oral and oropharyngeal tumors are diagnosed at an advanced stage, which contributes to a survival rate of only five years. Even in developed countries, the rates have not improved in recent decades compared to other cancers19, 20.
Treatment for Oral Cancer
Although oral cancer represent a third of all malignant tumors of head and neck, their treatment options were not different in the last three decades. Frequently used therapies for the treatment of oral cancer include surgery, radiotherapy and chemotherapy. These treatments consist of removing the tumor completely and inducing lethal changes on both neoplastic and adjacent normal cells, through radiation and chemotherapy drugs. Choosing the most appropriate therapy depends on factors such as the tumor size, tumor staging, proximity to anatomical structures, involvement of the lymphatic chain, age and patient cooperation, and the histological subtype of the lesion20, 21.
Surgery is the treatment of choice for squamous cell carcinoma in early stages. In later stages of these lesions, the use of adjuvant radiotherapy or chemotherapy is recommended. The results obtained by these treatments indicate cure rates between 72 and 93% for tumors in early stage of progression. However, although they provide a good prognosis, these therapies cause post-treatment complications that can affect aesthetics and function of the regions adjacent to the tumor, causing a sharp decrease in quality of life of patients22.
Even with the most modern procedures for surgical reconstruction, one of the consequences after the treatments is the occurrence of facial disfigurement, promoting serious cosmetic and functional defects. In such cases, severe psychological and emotional effects can also affect patient's recovery, who often need an extended professional multidisciplinary follow-up to return to normal daily life8, 23.
Traditional treatments for oral cancer may lead to a broad spectrum of adverse effects to the maxillomandibular complex and associated structures. The most frequent complications related to radiotherapy are: mucositis, candidiasis, hyposalivation, radiation caries, dysphagia, taste loss, muscular trismus, vascular changes and osteoradionecrosis, which is the most serious complication24, 25. An exacerbation of infections such as periapical and periodontal diseases, and severe mucositis may, occasionally, demand an adjustment of treatment or the discontinuation of radiotherapy or chemotherapy, demonstrating that oral complications should be prevented or minimized.
Photodynamic Therapy
Based on the current therapeutic possibilities for the treatment of mouth neoplasms, several studies have demonstrated the use of photodynamic therapy (PDT) as an alternative for the treatment of a variety of malignancies, including oral cancer. In comparison with radiotherapy or chemotherapy, PDT is generally safer for the surrounding normal tissues because the photosensitizers are preferentially accumulated in tumor cells26,27. The great advantage in using this therapy is the reduction of side effects compared with other treatment modalities. Moreover, the application of PDT in the public health service has aroused great interest because, since PDT do not demand hospitalization, it presents lower operating cost compared to surgery and chemotherapy28.
Mechanisms of photochemical action
The principle of PDT is based on the use of two individually non-toxic components that are combined to induce cellular and tissue effects in an oxygen-dependent manner. The first component is the photosensitizer, a photosensitive molecule that has affinity to neoplastic cells. The second component is a light source (laser or light emitting diodes - LED), with specific wavelength which activates the photosensitizer. This drug then transfers the energy from light to molecular oxygen, generating reactive oxygen species (ROS). These reactions occur at sites of immediate accumulation of the photosensitizer that absorbed the light. Therefore, the biological responses are activated only in specific areas of the tissue that were exposed to light29.
Photodynamic therapy is a multistep process involving the selective absorption of a photosensitizing drug by the tumor tissue, followed by the irradiation of the neoplastic lesion by a light source with specific wavelength. This radiation is capable of triggering photochemical reactions that generates singlet oxygen (1O2) and other reactive oxygen species (ROS). ROS generated by this process cause cytotoxic effects on tumor cells leading to tumor destruction. The drugs clinically used in PDT may bind the plasma membrane, intracellular membranes of the endoplasmic reticulum, mitochondria, lysosomes, or combinations of these sites. Once photosensitizers do not have the nucleus is a primary site to bind, such anti-cancer therapy can be considered less genotoxic if compared with radiotherapy or chemotherapy. The extent photodynamic of damage and cytotoxicity after PDT in vivo is multifactorial and depends on the type of photosensitizing agent used, its intracellular location, the time between the drug administration and the irradiation, as well as the different conditions of light irradiation. Moreover, the type of tumor and its level of oxygenation are crucial for the induction of photodynamic damage30.
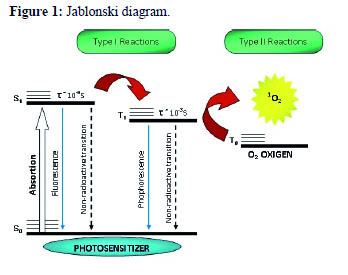
Photodynamic effect can be demonstrated through two mechanisms called Type I and Type II. The processes involved in both mechanisms are shown in the Jablonski diagram (Figure 1).
After absorbing a photon, the molecule of the photosensitizer (PS) changes from the ground state (So) to the singlet excited state (S1). From this excited state, the PS can return to the ground state emitting a photon of energy through non-radioactive and radiative processes (fluorescence). The PS, in the excited state, can also reverse spontaneously its spin through the process of intersystem crossing and go from S1 to the triplet state (T1). Once formed, the T1 can undergo decay to the ground state via non-radioactive and radioactive processes (phosphorescence)30.
The type I mechanism involves reactions of electron transfer between the molecule of the PS in their excited state S1 or T1 and the substrate. This process results in the formation of ion radicals that tend to react instantly with oxygen, producing a mixture of highly reactive oxygen intermediates like superoxide radical (•O2), hydrogen peroxide (H2O2) and hydroxyl radical (•OH), which oxidize a wide variety of biomolecules31.
The type II mechanism is characterized by reactions of energy transfer between the PS in the T1 state and molecular oxygen, which is also a triplet in the ground state (T0). These reactions lead to the formation of singlet oxygen (1O2), which is able to rapidly oxidize cell constituents and organelles resulting in the death of cancer cells31.
Both mechanisms may occur simultaneously. The proportion between them is highly influenced by the PS, the substrate, the oxygen concentration and the binding of PS to the substrate. However, type II mechanism seems to be more efficient because it has a higher rate constant than charge transfer reactions (type I mechanism). Consequently, the energy transferred to other compounds that can compete with oxygen is less important and the type II mechanism is often dominan31.
The singlet oxygen produced by a photochemical reaction is a highly reactive species, with electrophilic character. This ROS is able to induce oxidation of cellular molecules. Protein and unsaturated lipids are their main targets, resulting in irreversible damage to cellular organelles and cancer cells death. This reactive form of oxygen can also cause damage to the tumor vasculature, resulting an indirect form of tumor cell death, by hypoxia or starvation. In addition, damages in the cell membranes cause the release of inflammatory mediators and immunological factors, which initiate a cascade of events responsible for cell death in several other tumor cells29.
Singlet oxygen has a short lifetime in biological systems (<0.04 μs) and a diffusion potential with a small radius of action (0.02 μm). Therefore, tissue damage resulting from photodynamic treatment is restricted to cancer cells and the penetration depth of light used to activate the photosensitizer29.
Since the interaction of singlet oxygen with organic molecules is not specific, any macromolecule within the cell may be a potential target for PDT. Therefore, due to the possible multiple targets, tumor cells hardly develop resistance to this treatment, which is one of the advantages of photosensitization. Other advantages of PDT refer to the possibility of repeating the procedure several times without the risk of cumulative toxicity in tissue, besides the possibility of retreatment with surgery or radiation therapy when such therapies are needed32.
Biological mechanisms involved in the Photodynamic Therapy
Dolmans et al. (2003) describe that the generation of reactive oxygen species (ROS) leads to tumor destruction by three main biological mechanisms: (1) direct destruction of tumor mass by the action of ROS, (2) damage to the tumor vasculature, creating areas of hypoxia in tumor masses and (3) reduction of tumor mass in a secondary phase as a result of the activation of the immune system by necrosis and / or apoptosis31.
ROS formed after the application of PDT are ionic molecules with high chemical activity and the potential to react with any biomolecule, stabilizing these chemical species. Due to the low diffusivity of ROS through cellular compartments, the cellular sites affected by PDT will be those where there was a selective accumulation of the photosensitizing drugs. Moreover, cellular sites where there is accumulation of the drugs are associated to physical and chemical characteristics of each class of drugs33.
Several histological studies have demonstrated that the application of PDT promotes tumor cells death by both mechanisms: necrosis and apoptosis. The large amount of ROS after PDT promotes numerous changes in the tumor cells. Tumor clones death may derive from mechanisms of apoptosis and necrosis, depending on the subcellular regions affected and on the intensity of the stimulus34.
Cell death by necrosis, in general, is related to the presence of an aggressive agent of high intensity, as the ROS formed after PDT. In this situation, the basic cellular mechanisms of defense and are ineffective. As striking features of necrosis, it is important to highlight the functional changes in different subcellular compartments, specially the disruption of biological membranes causing the release of cell content. In association with this process, membrane phospholipids are released, triggering an intense inflammation through the lipooxigenase pathway35.
Another cell death pathway is apoptosis, that occurs in both physiological and pathological events. The morphological events observed in cells undergoing apoptosis are chromatin condensation, packaging and release of cytoplasmic fragments and organelles in microvesicles wrapped in plasma membrane (the apoptotic bodies). The vesicles released are phagocytized by neighboring cells, triggering an inflammatory process in lower intensity compared to necrosis. Such lower refers to the non-release of membrane phospholipids. After PDT, the induction of apoptosis is less frequent than necrosis in cancer tissue36.
The characterization of these two cell death processes, necrosis and apoptosis, can be performed with biochemical or morphological analysis. Numerous studies discuss the advantages and disadvantages of the occurrence of tumor cell death by each mechanism. This discussion is based on the stimulation or not of the immune system. Necrosis stimulates a greater immune response than apoptosis. Although the immune stimulation can induce adverse inflammatory events to patients, it can also promote the development of specific immune response against the treated tumors and regulate the tumor volume37.
The second mechanism of tumor control post-PDT is the induction of vascular changes in the tumor areas. The major events reported are thrombosis and vessels occlusion, which promote the reduction and the collapse of the blood flow and consequent hypoxia in the tumor areas supplied by these vessels. The presence of inflammatory cells, platelet aggregation, vascular endothelial injury and necrosis areas enhance the development of thrombotic events after photodynamic therapy32.
The third mechanism involved in the destruction of tumor masses is the action of the immune system against cancer cells. Contrary to other cancer therapies, PDT has an immunostimulating effect. A high activity of immune cells was observed in tumors treated with PDT. Some authors also describe a systemic antitumor protective effect after PDT, related to the selection of memory T lymphocytes against tumor clones treated at the primary site of cancer37.
Photosensitizer Drugs
The photosensitizers (PS) are the main elements in the performance of PDT. These drugs are activated by the light energy to generate reactions that culminate in the formation of highly reactive chemical species, which promote the destruction of target cells29.
A large number of PS has been clinically tested for PDT, but the results still suggest further research to develop more efficient PS. The prerequisites for an ideal PS include chemical purity, selectivity for tumor cells, chemical and physical stability, short time interval between the administration and the maximum accumulation in tumor tissues, activation at wavelengths with excellent tissue penetration and rapid excretion. The photophysical parameters desired are a high quantum yield of singlet oxygen as well as a high lifetime of the triplet state, because, in this case, the production of singlet oxygen is more effective38.
The major classes of clinically used PS are shown in (Table 1), each of these classes of drugs exhibit different photophysical and photochemical properties.
Several factors must be observed to choose the best PS: toxicity and carcinogenicity of the drugs, selectivity for target cells; possible side effects after exposure to white light, route of administration, costs, drugs elimination, wavelength for activation and clinical effectiveness. A subjective combination of all these factors must be performed to justify the selection of the most appropriated PS among the various treatment options32, 39.
Clinical application of PDT in Oral Cancer
PDT is already approved for clinical use in the United States, Canada, Russia, Japan and some European Union countries. In Brazil, this therapy is allowed for clinical research. For tumors of the mouth, the most widely used PS are Photofrin® (a porphyrin derivative) and Foscan® (an ALA derivative). More than 1500 cases of treatment of head and neck tumors with PDT have been reported. They were followed from 1990 to 2006. Clinically, this therapy has proven effective especially in cases of early tumors and precancerous lesions26.
PDT is already approved for clinical use in the United States, Canada, Russia, Japan and some European Union countries. In Brazil, this therapy is allowed for clinical research. For tumors of the mouth, the most widely used PS are Photofrin® (a porphyrin derivative) and Foscan® (an ALA derivative). More than 1500 cases of treatment of head and neck tumors with PDT have been reported. They were followed from 1990 to 2006. Clinically, this therapy has proven effective especially in cases of early tumors and precancerous lesions26.
BIEL (2007), on a meta-analysis article, reports that among 518 patients with early tumors of head and neck (T1 or T2), 462 (89.1%) showed complete remission after a single treatment with PDT. In the same paper, the author describes that, in a 16-year follow-up of 171 patients presenting larynx tumors treated with PDT, there was complete remission and no recurrence. These results are very encouraging for the use of PDT in the treatment of oral cancer, but follow-up studies of treated patients are still needed to establish the effectiveness of this therapy24.
Although the PDT presents great advantages in the reduction of side effects commonly described after the traditional therapies for cancer, as described above, it can cause some other side effects that are mainly related to different patterns of skin phototoxicity26, 27.
CONCLUSION
Oral cancer accounts for about one third of all malignant tumors of head and neck and presents major challenges to optimize treatment options that limit morbidity and maximize the chances of cure. In this context, photodynamic therapy in has aroused great interest for its clinical application in the treatment of tumors in the oral cavity. The drastic reduction of the adverse effects of therapy, the conservative intervention and its simplicity are the major attractions of PDT. Currently, great hopes are placed in this method for treatment of cancer, but more careful long term studies are required to establish the real efficacy of this treatment protocol.
ACKNOWLEDGES
This article was part of the dissertation in Health Sciences conducted by Leandro Santos Bicalho in the Faculty of Health Sciences, University of Brasilia. The authors appreciate the financial support from MCT, FINEP, CNPq, CAPES, FAP-DF.
INTEREST CONFLICT
The authors declare there is no proprietary, financial, professional or other personal interest of any nature that could influence the information in this article.
REFERENCES
1. Brasil. Ministry of Health. National Cancer Institute - INCA. Estimates of cancer incidence and mortality. Rio de Janeiro: INCA. 2010. [ Links ]
2. Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010 Jun 17. [ Links ]
3. Petersen PE. Oral cancer prevention and control--the approach of the World Health Organization. Oral Oncol 2009; 45: 454-460. [ Links ]
4. Knowles M, Selby P. Introduction to the Cellular and Molecular Biology of Cancer Oxford-Bioscience. 2005. [ Links ]
5. Molinolo AA, Amornphimoltham P, Squarize CH, Castilho RM, Patel V, Gutkind JS. Dysregulated molecular networks in head and neck carcinogenesis. Oral Oncol 2009; 45: 324-334. [ Links ]
6. Todd R, Donoff RB, Wong DT. The molecular biology of oral carcinogenesis: toward a tumor progression model. J Oral Maxillofac Surg 1997; 55:613-623. [ Links ]
7. Hursting SD, Slaga TJ, Fischer SM, DiGiovanni J, Phang JM. Mechanism-based cancer prevention approaches: targets, examples, and the use of transgenic mice. J Natl Cancer Inst 1999 3; 91: 215-225. [ Links ]
8. Shah JP, Gil Z. Current concepts in management of oral cancer--surgery. Oral Oncol 2009; 45: 394-401. [ Links ]
9. Tsantoulis PK, Kastrinakis NG, Tourvas AD, Laskaris G, Gorgoulis VG. Advances in the biology of oral cancer. Oral Oncol 2007; 43: 523-34. [ Links ]
10. Warnakulasuriya S. Global epidemiology of oral and oropharyngeal cancer. Oral Oncol 2009; 45: 309-316. [ Links ]
11. De Camargo Cancela M, Voti L, Guerra-Yi M, Chapuis F, Mazuir M, Curado MP. Oral cavity cancer in developed and in developing countries: population-based incidence. Head Neck 2009; 32: 357-367. [ Links ]
12. Kojima A, Maeda H, Sugita Y, Tanaka S, Kameyama Y. Human papillomavirus type 38 infection in oral squamous cell carcinomas. Oral Oncol 2002; 38: 591-596. [ Links ]
13. Warnakulasuriya S. Living with oral cancer: epidemiology with particular reference to prevalence and life-style changes that influence survival. Oral Oncol 2010; 46: 407-410. [ Links ]
14. Garavello W, Lucenteforte E, Bosetti C, La Vecchia C. The role of foods and nutrients on oral and pharyngeal cancer risk. Minerva Stomatol. 2009; 58: 25-34. [ Links ]
15. Goldenberg D, Brooksby C, Hollenbeak CS. Age as a determinant of outcomes for patients with oral cancer. Oral Oncol 2009; 45: 57-61. [ Links ]
16. Lucenteforte E, Garavello W, Bosetti C, La Vecchia C. Dietary factors and oral and pharyngeal cancer risk. Oral Oncol 2009; 45: 461-467. [ Links ]
17. Meurman JH. Infectious and dietary risk factors of oral cancer. Oral Oncol 46: 411-413. [ Links ]
18. Popovic B, Jekic B, Novakovic I, Lukovic L, Konstantinovic V, Babic M, et al. Cancer genes alterations and HPV infection in oral squamous cell carcinoma. Int J Oral Maxillofac Surg. Jun 23. [ Links ]
19. Chandu A, Smith AC, Rogers SN. Health-related quality of life in oral cancer: a review. J Oral Maxillofac Surg 2006; 64: 495-502. [ Links ]
20. Lung T, Tascau OC, Almasan HA, Muresan O. Head and neck cancer, treatment, evolution and post therapeutic survival - Part 2: a decade's results 1993-2002. J Craniomaxillofac Surg 2007; 35: 126-131. [ Links ]
21. Kalavrezos N, Bhandari R. Current trends and future perspectives in the surgical management of oral cancer. Oral Oncol; 46: 429-432. [ Links ]
22. Choong N, Vokes E. Expanding role of the medical oncologist in the management of head and neck cancer. CA Cancer J Clin 2008; 58: 32-53. [ Links ]
23. Crozier E, Sumer BD. Head and neck cancer. Med Clin North Am 2010; 94: 1031-1046. [ Links ]
24. Biel MA. Photodynamic therapy treatment of early oral and laryngeal cancers. Photochem Photobiol 2007; 83: 1063-1068. [ Links ]
25. Menzin J, Lines LM, Manning LN. The economics of squamous cell carcinoma of the head and neck. Curr Opin Otolaryngol Head Neck Surg 2007; 15: 68-73. [ Links ]
26. Hopper C, Niziol C, Sidhu M. The cost-effectiveness of Foscan mediated photodynamic therapy (Foscan-PDT) compared with extensive palliative surgery and palliative chemotherapy for patients with advanced head and neck cancer in the UK. Oral Oncol 2004; 40: 372-382. [ Links ]
27. Luksiene Z. Photodynamic therapy: mechanism of action and ways to improve the efficiency of treatment. Medicina (Kaunas). 2003; 39: 1137-1150. [ Links ]
28. Castano AP, Mroz P, Hamblin MR. Photodynamic therapy and anti-tumour immunity. Nat Rev Cancer 2006; 6: 535-545. [ Links ]
29. Nyst HJ, Tan IB, Stewart FA, Balm AJ. Is photodynamic therapy a good alternative to surgery and radiotherapy in the treatment of head and neck cancer? Photodiagnosis Photodyn Ther 2009; 6: 3-11. [ Links ]
30. Allison RR, Bagnato VS, Sibata CH. Future of oncologic photodynamic therapy. Future Oncol; 6: 929-940. [ Links ]
31. Dolmans DE, Fukumura D, Jain RK. Photodynamic therapy for cancer. Nat Rev Cancer 2003; 3: 380-387. [ Links ]
32. Robertson CA, Evans DH, Abrahamse H. Photodynamic therapy (PDT): a short review on cellular mechanisms and cancer research applications for PDT. J Photochem Photobiol B 2009; 96: 1-8. [ Links ]
33. Ord RA, Blanchaert RH, Jr. Current management of oral cancer. A multidisciplinary approach. J Am Dent Assoc 2001; 132: 19S-23S. [ Links ]
34. Buytaert E, Dewaele M, Agostinis P. Molecular effectors of multiple cell death pathways initiated by photodynamic therapy. Biochim Biophys Acta 2007; 1776: 86-107. [ Links ]
35. Krammer B. Vascular effects of photodynamic therapy. Anticancer Res 2001; 21: 4271-4277. [ Links ]
36. Bobrov N, Cavarga I, Longauer F, Rybarova S, Fedorocko P, Brezani P, et al. Histomorphological changes in murine fibrosarcoma after hypericin-based photodynamic therapy. Phytomedicine 2007; 14: 172-178. [ Links ]
37. Dougherty TJ, Gomer CJ, Henderson BW, Jori G, Kessel D, Korbelik M, et al. Photodynamic therapy. J Natl Cancer Inst 1998; 90: 889-905. [ Links ]
38. Allison RR, Sibata CH. Oncologic photodynamic therapy photosensitizers: a clinical review. Photodiagnosis Photodyn Ther 2010; 7: 61-75. [ Links ]
39. Biel MA. Photodynamic therapy of head and neck cancers. Methods Mol Biol 2010; 635: 281-293. [ Links ]