I. Introduction
According to statistics from the Water Footprint Network (WFN), global average water consumption is around 1240 m3 per person per year. In countries, such as Spain or the United States, the consumption is higher reaching 2500 m3, whereas in China is less than 700 m3 [1]. In Colombia, the average consumption in 2007 was close to 900 m3 [1], of which approximately 90 % became wastewater [2] that was discharged into water bodies with little to none previous treatment.
Aluminum sulphate is the industrial coagulant traditionally used for water treatment and water purification [3]. This coagulant, under optimal conditions, can achieve between 90 % and 99 % efficiency on removing microorganisms [4]. However, it has drawbacks, such as the fact that the residual aluminum resulted from the process of coagulation-flocculation has a high eco-toxicological and economic impact when is discarded into the environment through the treated sludge, and the high cost for communities involved in its import and transport [5]. For these reasons, it is necessary to find new environmentally friendly coagulant alternatives. Recent studies have shown that natural coagulants may be better economically and less polluting [5]. By introducing this type of coagulant on a wastewater treatment plant, it may generate economic development and reduce environmental impact on communities.
In the scientific literature, some natural coagulants have a high percentage of turbidity, color, and organic matter removal. Almendárez et al. [6] showed the effectiveness of the Cochifloc polymeric coagulant on waters of Lake "Piedras Azules" in Managua. In that study, the authors compared turbidity removal after applying the polymer Cochifloc, aluminum sulphate, ferric chloride, and Quimifloc (a synthetic polymer). They showed that the effectiveness of turbidity removal of Cochifloc was superior, achieving 91 %.
Carpinteyro [7] evaluated the coagulant capacity of two natural polymers: nopal mucilage and gum seed mezquite. The natural polymers were used on domestic and cosmetic industry wastewater, finding that the biopolymers have better performance on treating domestic wastewater with the lowest dose (25 mg/l), removing between 44 % and 52 % of the Chemical Oxygen Demand (COD).
Vázquez et al. [8] performed coagulation tests on samples of domestic wastewater using nopal as a natural coagulant, and combining it with aluminum sulphate as coagulation mediator. Using the natural coagulant individually, results show a turbidity removal of 58 %, total suspended solids (TSS) removal of 33.4 %, and COD removal of 37.5 %. When aluminum sulphate was applied, the results for turbidity, SST and COD were 92 %, 95.6 % and 50 %, respectively. Suárez et al. [9] found that thermal water produced significantly better removals, and had a better economic performance for the Advanced Primary Treatment (APT) than those achieved with ferric chloride. Furthermore, Guzmán et al. [10] reported the use of plant origin natural coagulants to remove turbidity and color from untreated sewage water. Yin et al. [11] found that most plant extracts used for removal of turbidity in water are efficient, and produce less sludge compared to aluminum sulphate.
The use of natural coagulants stands as an environmental friendly technology, which has greater biodegradability than chemical coagulants [12]. Thermal water is a natural renewable resource, abundant in the region of Manizales city (Caldas, Colombia), and can be used as a natural coagulant in the process of coagulation-flocculation due to its iron and aluminum content [13].
This work aimed at studying the potential use of thermal water as a natural coagulant on the treatment of domestic wastewater (DWW). The DWW is characterized by high organic load, high Chemical Oxygen Demand (COD), high Biochemical Oxygen Demand (BOD), and a negative effect on water bodies. These pollutants decrease dissolved oxygen, preventing the normal development of living organisms such as fish, invertebrates, and other species that inhabit rivers, streams, and lakes [14].
II. Materials and methods
A. Wastewater
Domestic wastewater was collected from a sewer drain outlet of Manizales city (Caldas, Colombia), which has approximately 368,000 inhabitants in the urban area [15]. Sampling was done following instructions from the Institute of Hydrology, Meteorology and Environmental Studies (IDEAM by its Spanish initials) Code TIO 187 [16]. Immediately after collection, the samples were sent to the laboratory for analysis and trials in order to prevent natural decomposition processes, such as hydrolysis and acidification, which may modify the original conditions of the samples [17].
B. Coagulants
Thermal water samples were collected at the Nevados National Park (Snowcapped Mountain Park), and were used as a natural coagulant. The samples were taken directly from a storage tank, placed in glass containers, and stored at 4 °C until they were used. Aluminum sulphate A12(SO4)3 solid type B was used as a conventional coagulant. This coagulant is the same used by the aqueduct of Manizales for water purification. The technical specifications of the product are shown in Table 1.
C. Analysis of physicochemical parameters
We followed the guidelines of the Standard Methods edition 22 [19] to measure the physical and chemical parameters of thermal water, and untreated and treated domestic wastewater. Colorimetric parameters such as color (ST 2120 C), aluminum (ST 3500-AI D), total iron (ST 3500-Fe D), nitrates (ST 4500-NO3 b), nitrites (ST 4500-NO2 B), sulfate (ST 4500-SO4 E), and phosphates (ST 4500-P D), were measured with a spectrophotometer Cary( 50 UV-VIS. Potentiometric and titration parameters such as pH (ST 4500-H B), conductivity (ST 2510 B), total alkalinity (ST 2320 b), total hardness (ST 2340 C), calcium hardness (ST 2340 C) , calcium (ST 3500-Ca D), magnesium (ST 3500-Mg E), chlorides (ST 4500-Cl B), COD (ST 5220-O D) , and BOD (ST 5210-O B) were measured with an automatic titration Metler Toledo DL 50. Turbidity (ST 2130B) was measured with a turbidimeter HACH 2100P, and total suspended solids (ST 2540D) with a scale Metler Toledo XP504. In order to evaluate the effectiveness of the process, removal percentages were calculated using the equation (1)[20]:
D. Coagulation-Jar test
For the coagulation-flocculation process, a solution of aluminum sulphate 2 % (w/v) was prepared using distilled water as solvent, following the methodology reported by Arboleda [3]. The test consisted of a series of beakers or jars representing the coagulation-flocculation process at the treatment plant, and at the end, different parameters were evaluated to characterize its operation. This procedure was performed according to the Colombian Technical Standard NTC 3903 [21]. The equipment used was a Phipps & Bird jar test (00702) with rounded jars of 2 liters each, in which the same amount of waste water sample was used. The test began with a rapid mixing at 300 rpm [22], the doses were added to each beaker during rapid stirring, and almost instantly starting counting a minute [3]. Rapid mixing was followed by the flocculation process at 30 rpm for 20 minutes. After this time, the stirring process was stopped, and sedimentation was allowed for 30 minutes [23]. Once the coagulation-flocculation process finished, samples were taken from the supernatant of each jar to measure turbidity and COD [24]. The jar with the lowest parameter values was defined as the optimal coagulant dose. The same procedure was used for both aluminum sulphate and thermal water.
Concentrations used for testing jars with aluminum sulphate were: 30 mg/l, 50 mg/l, 70 mg/l, 90 mg/l, 110 mg/l, 130 mg/l, 135 mg/l, 140 mg/l, 145 mg/l, 150 mg/l, and 155 mg/l. These doses were selected based on previous experiences from Water Quality laboratory, Aguas de Manizales S.A. E.S.P., and reports such as Guida et al. [25] and Haydar et al. [22]. These reports found an optimal doses of aluminum sulfate of 150 mg/l and 100 mg/l, respectively for the treatment of domestic wastewater in Naples (Italy) and Sheikhupura (Pakistan).
The concentrations used for testing jars with thermal water were: 10 ml/l, 15 ml/l, 20 ml/l, 25 ml/l, 30 ml/l, 35 ml/l, 40 ml/l, and 45 ml/l. These doses were selected following Suárez et al. [17], who found that an acceptable range of optimal dose of thermal water coagulant for municipal wastewater was between 10 ml/l and 30 ml/l.
The most effective coagulant was the one with the highest percentages of removal in each of the evaluated parameters [26]. All the analyzes were performed in triplicate, and results were expressed with the corresponding standard deviation. The variance test was calculated using one-way ANOVA, with statistical significance of p<0.05.
III. Results and discussion
A. Thermal water characterization
Thermal water physicochemical characterization is presented in Table 2. The characterization provides important information about the potential that thermal water has as a natural coagulant due to the presence of sulfates and aluminum.
Table 2 shows that high concentrations of sulfate (near to 4900 mg/l) and aluminum (240 mg/l) can be found in thermal water, demonstrating that these compounds can be responsible for the coagulation capacity of this type of water. Aluminum sulphate has a high coagulation capability and is responsible for performing the vast majority of coagulation-flocculation treatments on drinking water and wastewater [3]. This coagulant capacity is given by the high presence of iron, calcium and magnesium ions. In the industry, calcium chloride and calcium hydroxide are known as calcium based coagulants, magnesium chloride is known as magnesium based coagulant, and ferric chloride as iron based coagulant [26].
Table 3 presents the characterization of DWW. The most important parameters to consider are total suspended solids (BOD) and organic compounds (COD), due to the high environmental impact generated when they are discharged into water bodies, creating not only environmental but economic disadvantages for those who does not comply with the current Colombian legislation (Decree 3930 of 2010) [27]; therefore, most wastewater management facilities are designed to remove them [2].
Table 3 Domestic wastewater characterization

1 ± Method Uncertainty
2 ± Three different data was used to calculated standard deviation
According to Montoya et al. [28], turbidity in water has been widely applied as a quality criterion because high turbidity appearance generates people rejection. DWW has a turbidity of 232 UNT (Table 3) due to the high presence of solid material, especially suspended solids. This parameter is very important in the coagulation processes, mainly because it is easy to measure and sensitive to coagulation processes [29]. Furthermore, when the process removes a good percentage of turbidity from wastewater, it removes a large part of organic material, microorganisms and color [30].
On the other hand, the DWW characterized is biodegradable, which is demonstrated by the value of the BOD/COD ratio that is equal to 0.4. According to the Manual for Urban Wastewater Treatment [31], it is feasible to perform advanced primary process for the treatment of DWW, in other words, to conduct a coagulation and flocculation process [32].
B. Optimal dose using aluminum sulphate
The effect of applying aluminum sulphate coagulant (2 % w/v) on turbidity and COD is shown in Figures 1 and 2, respectively. As the dose of aluminum sulphate rose, the removal of turbidity and COD increased, achieving maximum removal at 140 mg/l dose, equivalent to an application of 22.1 mg of aluminum per liter of wastewater. Doses higher than 140 mg/l resulted in removal decrease, which is related to the optimal dose curves typically found in drinking water treatment [3]. This phenomenon can be ascribed to the re-stabilization of the colloidal particles, which occurs when the coagulant is overused, or values above optimal are obtained [33].

Fig. 1. Effect of applying different doses of aluminum sulphate coagulant (2 % w/v) on turbidity for untreated domestic wastewater.

Fig.2. Effect of applying different doses of aluminum sulphate (2 % w/v) on COD for untreated domestic wastewater.
The response variables, turbidity and COD, exhibit similar behavior. With a dose of140 mg/l of aluminum sulphate, the final turbidity was 4.4 NTU, and COD was 134.6 mg/l, obtaining removals of 98.12 % and 81.5 %, respectively. These results are higher than those reported by Haydar et al. [22], who using aluminum sulphate on domestic wastewater found turbidity and COD removals of 97 % and 53.3 %, respectively. Guida et al. [25] reported similar values to those found in this work, with COD removal of 59 % using the same coagulant. Additionally, it was found that BOD increased from 323 mg/l in untreated domestic wastewater to 101 mg/l in wastewater treated with aluminum sulphate, thereby obtaining a 68.7 % of removal.
According to the Colombian Resolution 0631 of 2015, the parameters BOD and COD must have a maximum allowed value of 180 mg/l and 90 mg/l, respectively [34]. Therefore, at this stage, the process complies with the resolution of the maximum allowable value for COD, and with an 89 % of the maximum permissible value of BOD. To comply with the Colombian law, it is necessary to continue with the next steps in the treatment process.
The BOD/COD ratio for wastewater treated with aluminum sulphate was 0.75, indicating high biodegradability. This means that the treatment decreased the content of organic matter, bringing environmental benefits when discharged [32].
In conclusion, this study found that the optimal dose of aluminum sulphate for untreated domestic wastewater in the city of Manizales is close to 140 mg/l. The ANOVA indicated significant differences between all doses evaluated to remove turbidity and COD (GL 32; p<0.05; F 2237). The Tukey test showed that, although there are significant differences between doses of 140 mg/l and 150 mg/l, these doses behave statistically similar to each other, and different to the other treatments, obtaining the best turbidity removal values.
C. Optimal dose determination using thermal water as a natural coagulant
All doses of thermal water had positive removal values of turbidity and COD (Fig. 3 and 4). As the thermal water dose increased, removal also increased, reaching stability values at 20 ml/l dose. For thermal water doses of 10 ml/l and 35 ml/l, turbidity parameter removals of 60.2 % and 98 %, respectively, were found. For thermal water doses of 10 ml/l and 35 ml/l, COD removal of 7.5 % and 41.1 % were found, equivalent to an application of 8.4 mg of aluminum per liter of wastewater.

Fig. 3 Effect of applying different thermal water doses as a coagulant on turbidity for untreated domestic wastewater.
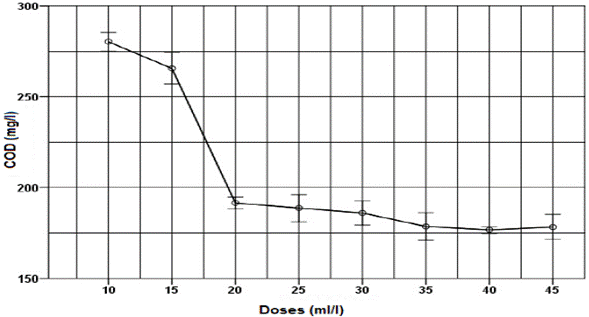
Fig. 4. Effect of applying different thermal water doses as a coagulant on COD for untreated domestic wastewater.
The response variables, turbidity and COD, exhibit similar behavior after thermal water application. As thermal water dose increased, both turbidity and COD decreased until a dose of 35 ml/l, where an asymptotic behavior was reached, in which even if an excess of coagulant was added, higher removals were not obtained; this is a typical behavior of a sweep coagulation [3]. Optimal dose of thermal water was found at 35 ml/l, obtaining turbidity and COD values of 4.4 NTU and 178.6 mg/l, respectively. Similarly, for the response variables, turbidity and COD, removals of 98.1 % and 75.4 % were obtained respectively. These results are higher to those reported by Bhuptawat et al. [23] and Subramonian et al. [20], who found COD removals of 50 % and 36.2 % for the natural coagulants Moringa oleifera and Cassia obtusifolia, respectively. In addition, we found that the BOD decreased from 323 mg/l in the untreated wastewater to 91 mg/l in the wastewater treated with thermal water, obtaining a 71.8 % removal.
COD of 178.6 mg/l and BOD of 91 mg/l after using thermal water as a coagulant are almost fully compliant with the maximum permissible values allowed by the Colombian law for domestic wastewater (COD of 180 mg/l, and BOD of 90 mg/l) [34]. This means that additional processes help to improve the quality of these waters, reducing pollution and complying with increasingly stringent legislation.
The BOD/COD ratio ofwastewater treated with thermal water was 0.5, indicating high water biodegradability. Despite the fact that the biodegradability obtained is lower in comparison with water treated with aluminum sulphate, environmental benefits were also obtained in the final disposal reducing the organic matter initially found [32].
The ANOVA indicated significant differences between all doses evaluated for turbidity removal and COD (GL 23; p <0.05; F 4223). The Tukey test showed that although there are significant differences among 30 ml/l, 35 ml/l and 40 ml/l doses, they behave statistically similar to each other and different to other treatments. The dose of 45 ml/l is statistically similar to 35 ml/l. However, if one considers to reduce the consumption of the coagulant in order to economize the thermal water, it is concluded that the optimal dose is close to 35 ml/l.
Turbidity and COD final parameter values obtained with optimal doses of conventional coagulant (aluminum sulphate) and natural coagulant (thermal water) were similar, with differences of 0.02 % in turbidity and 14.5 % in COD.
A preliminary analysis on coagulant costs was conducted for all the methods used, taking into account solid aluminum sulphate and thermal water commercial prices (January 31, 2015) [13].
Table 4 Preliminary economic comrarison on the costs of coagulants for DWW treatment evaluating two methods

1 Calculated based on initial COD (726.2 mg/l) and expected COD (180 mg/l) [34]
A preliminary cost analysis of the coagulation-flocculation stage as primary treatment found that using thermal water as a coagulant is 60.3 % cheaper than conventional coagulants, considering that similar efficiencies are achieved. Nevertheless, it is necessary to proceed with the subsequent stages of the treatment to comply with all the parameters required by the national legislation, which will generate an increase in the total treatment cost.
The cost of the coagulant used in the coagulation and flocculation stage of the DWW treatment using thermal water would be $0.049 Colombian pesos (Table 4), which means a 39 % decrease in treatment costs. This means that using thermal water as a natural coagulant is a sustainable alternative for domestic wastewater treatments.
IV. Conclusions
Once the characterization of the thermal water was done, high concentrations of sulphate (near 4900 mg/l) and aluminum (240 mg/l) were found, demonstrating that these compounds provided coagulant capacity for water treatment.
Final values for turbidity and COD in all samples were similar for both evaluated coagulants, conventional (aluminum sulphate) and natural (thermal water), with differences in turbidity (0.023 %) and COD (6.1 %). Similar removal optimal doses for both, aluminum sulphate and thermal water, were obtained. These optimal doses were equivalent to an application of 22.1 mg/l of aluminum using aluminum sulphate, and 8.4 mg/l using thermal water. This suggests that the coagulation power in aluminum sulphate is due to the action of aluminum, while in thermal water is due to other elements besides aluminum that have coagulant characteristics, such as calcium and magnesium.
Therefore, we can conclude that thermal water can be considered a technical alternative as a natural coagulant that removes pollutants from domestic wastewater with similar efficiency to aluminum sulphate. Finally, thermal water is a sustainable method for the treatment of domestic wastewater that can be used along with alternative coagulants in industries and businesses in the city of Manizales.

















