Introduction
At present Flow Cytometry (FCM) represents a diagnostic tool with high sensitivity and specificity. It is an indispensable tool recommended for routine clinical diagnosis, immunological classification, and post-treatment follow-up of patients with various types of diseases, including hematological malignancies, solid tumors, infectious diseases, and immune deficiency [1-3]. Analysis by FCM can be performed on different types of biological samples, such as bone marrow (BM), peripheral blood (PB), stem cell grafts, umbilical cord tissue biopsies, and body fluids, among others [1]. Body fluid samples require special attention due to cell viability diversity, limited sample volume and content variability, including cell concentration [4-5]. Furthermore, within less than six hours after their collection, these samples demonstrate decreased cell viability and diminished quality, presenting challenges for FCM analysis [4-7].
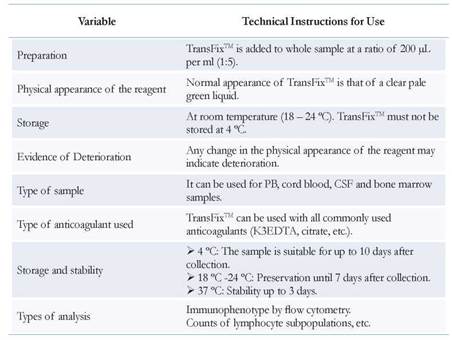
Consequently, over the last decade, use of commercially available cell stabilization solutions has been recommended. These solutions can preserve PB and cerebrospinal fluid (CSF) samples over time periods greater than one week after collection [4,8-9]. This stabilization preserves cellularity and integrity of both cell surface and intracellular antigens [9].
The Pontificia Universidad Javeriana flow cytometry service at the School of Sciences was established in 2008 to implement FCM in the study of stabilized body fluids. Since then at the time of collection samples are fixed with Transfix™ (Cytomark, Buckingham, UK) [2,4-5]. This service was then transferred to the Hospital Universitario San Ignacio, where FCM is currently performed. Today, special samples, such as cerebrospinal samples from pediatric and adult patients with various diseases that require correct classification and/or staging are evaluated. In our experience FCM has significantly contributed to the detection of tumor populations that have infiltrated various tissues such as the central nervous system, which is a clinical complication associated with increased aggressiveness and poor prognosis in patients with acute leukemia and lymphoma [2,4-5,7,10]. Compared with morphological studies FCM detects with greater sensitivity, identifying even very low tumor cell populations numbers (< 0.01 %). This is an advantage over morphological techniques, which cannot detect low numbers of malignant cells due to lower sensitivity. Moreover, tumor cells may be detected by FCM before onset of clinical symptoms [4,10-12].
Currently in Colombia, no results have been published related to FCM implementation for stabilized body fluids study. In the present work, we report the main findings of FCM body fluid evaluation over a period of six years at the Pontificia Universidad Javeriana and Hospital Universitario San Ignacio. These include technical recommendations for sample processing and analysis in the pre-analytical and analytical phases, absolute and relative cell counts of normal and tumor cell populations, sample volumes, types of diseases, in addition to clinical time point evaluation.
Implementation of our experience will be useful in our country for diagnosis in other cytometry services, as a fundamental tool in clinical practice.
Materials and Methods
We describe special fluid samples (n = 1070) processed and analyzed from June 2008 to June 2014 at Pontificia Universidad Javeriana and Hospital Universitario San Ignacio. Collected information included sex, age, and diagnosis, type of sample, volume, cellularity, tumor infiltration rate, and study justification (diagnosis, monitoring, relapse or progression).
Only body fluids samples that were fixed with Transfix™ (Cytomark, Buckingham, UK) were included in the study.
Results were analyzed with non-parametric statistics. Median, mean ± SD and value range were calculated using the SPSS software program (SPSS 19, Chicago, IL, USA).
Statistical significance was determined by Wilcoxon or Mann-Whitney U tests. P-values of p < 0.05* and p < 0.01** were considered statistically significant.
All individuals gave their written informed consent for obtaining the special samples and the study was approved by the Ethics Committe of the Hospital Universitario San Ignacio, Bogotá, Colombia.
Protocol used for processing, labeling, acquisition and analysis of special samples (body fluids) by FCM
The Colombian Consensus of FCM was established in 2008 [6]. It recommended body fluids samples (specifically CSF) be stabilized from the time of collection in tubes with Transfix™ (Cytomark, Buckingham, UK), a commercially available fixative agent (Barnett D. Patent WO 95/01796, 1995). This method has been validated in previous studies [2,4-5,13]. Transfix™ is a cellular stabilization reagent containing a buffer with an aliphatic aldehyde (AA) that fixes cells by cross-linking amino-acid residues. In addition, it contains heavy metal salts, which reduce excessive autofluorescence caused by AA. Hence, this fixative preserves samples for longer periods (up to 10 d) [4,13]. In the pre-analytical phase, Transfix™ was immediately added to the samples at the time of collection by the attending clinician. Once stabilized, samples were transported to the laboratory at 4 °C. It is recommended that specimens be incubated with Transfix™ for at least 18 h prior to processing, because this leads to higher leukocyte counts and improves tumor cell detection [13]. The detailed sample processing is shown in Figure 1.
To calculate the absolute number of each cell subpopulation, apply the following equation [4-5,14]:
where, the total number of beads added to the tube is calculated as the volume of beads added to the tube (e.g, 20 µL) multiplied by the beads concentration (beads/µL) (data are provided by the manufacturer). The volume of the sample is the total volume at the time of collection. The correction factor is assumed as equal to the initial volume of the sample (e.g., 5000 µL) divided by the volume of the concentrated sample used for staining in each tube (e.g., 100 µL) in such a way that, in the protocol described, the correction factor is: 5000 µL/100 µL = 50.
Analysis:
1. For the analysis, classify clusters of more than 25 events fulfilling the above criteria as positive, clusters of 10 events to 25 events as suspicious, and clusters of fewer than 10 events as negative [5].
2. If after the analysis no tumor infiltration is detected, the process should be repeated with the remaining sample volume (200 µL), with the same combination of antibodies in order to increase assay sensitivity. Conversely, if tumor infiltration is detected, and depending on the total number of cells/µL, perform an additional panel to characterize the cell population immunophenotype.
3. Generate the analysis report.
For simultaneous staining of membrane and intracellular antigens, a fixation and permeabilization kit (IntraStain-Dako) was used. First, the membrane antigens (step 7) were stained, and then the sample was incubated with 100 µL of fixative solution (Reagent A). Next, 100 µL of permeabilization solution (Reagent B) was added to the antibodies against the intracellular antigens. Last, incubation was performed for 30 min at room temperature in the dark, followed by washes with PBS + 0.5 % albumin (step 10 onwards).
Results
Between June 2008 to June 2014 at the Pontificia Universidad Javeriana and Hospital Universitario San Ignacio FCM core facility, 1070 special samples from 653 males (61 %) and 417 females (39 %) were studied retrospectively The mean age of the patients at the time of analysis was 37 years (range: 3 months old to 88 years).
The majority of the samples consisted of CSF (n = 932; 87.1 % of cases), followed by while pleural fluid samples (n = 95; 8.9 %). Broncho alveolar lavage (n = 24; 2.2 %), pericardial fluid (n = 8; 0.7 %), peritoneal fluid (n = 7, 0.7 %), ascites (n = 3, 0.3 %), and synovial fluid (n = 1; 0.1 %) represented fewer samples (Figure 2). Sample volumes were variable and ranged from 0.2 mL (in CSF samples) to 60 mL (in samples of pleural fluid) (Figure 3). All samples were stabilized with TransFix™ (Cytomark, Buckingham, UK) at the time of collection.

Fig. 2 Distribution of cases according to sample collected and analyzed by flow cytometry CSF: cerebrospinal fluid.

Fig. 3 Body fluid sample volumes (Mean + SD for each special sample). Volumes are in ml. CSF: cerebrospinal fluid.
According to clinical diagnosis most cases were acute leukemia (n = 606: B cell acute lymphoblastic leukemia, 495; T cell acute lymphoblastic leukemia, 59, acute myeloblastic leukemia, 23; biphenotypic acute leukemia, 6; and biclonal acute leukemia, 3). Other cases were Non-Hodgkin Lymphomas (NHL) (n = 317: B-NHL, 282; and T-NHL, 35), Hodgkin lymphoma (HL) (n = 8), chronic myeloid leukemia (CML) (n =12), myelodysplastic syndrome (MDS) (n = 5), multiple myeloma (MM) (n = 5), and solid tumors (ST) (n = 21). The remaining samples (n = 96) included patients with neurological symptoms, patients infected with human immunodeficiency virus (HIV+), and patients with autoimmune diseases, infections, and migraine, among other conditions (Figure 4). In all, 347 cases were analyzed at diagnosis (32 %), 682 were analyzed during clinical follow-up (64 %) and 41 were analyzed at relapse (4 %).

Fig. 4 Case distribution according to clinical suspicion. MDS: myelodysplastic syndrome; CML: Chronic Myelogenous Leukemia: NHL: Non-Hodgkin Lymphomas; HL: Hodgkin lymphoma; MM: multiple myeloma.

Fig. 4 Types of pathologies evaluated in body fluids samples (panels A, C and E) and tumor infiltration results (panels B, D and F). CSF: cerebrospinal fluid; HIV: human immunodeficiency virus, B-NHL: B-cell lymphoma, CML: chronic myeloid leukemia, AML: acute myeloid leukemia, B-ALL: acute lymphoblastic leukemia B; T-ALL: acute lymphoblastic leukemia T; LH: Hodgkin lymphoma.
Analysis of cerebrospinal fluid (CSF) samples
The total number of CSF samples analyzed was 932. The pathological condition most often identified was infiltration by B cell acute lymphoblastic leukemia (B-ALL) (54 % of cases), followed by B-NHL (26 %) (Figure 5A). Among the "other" category, of the total CSF samples, representing 6 % (63 samples) included cases diagnosed with meningoencephalitis, autoimmune diseases, infectious diseases or solid tumors.

Fig. 5 Representative result of bivariate dot plot histograms of a normal/reactive CSF sample from a B-cell lymphoma patient showing no CSF infiltration by multiparameter flow cytometry immunophenotyping. In this sample, CD3+/CD45+ T cells (green dots) and CD14+/CD45 + monocytes (black dots) were detected. Gray dots represent non leukocyte events (debris) and red dots correspond to fluorescent beads.
Of all the samples tested, 19 % demonstrated tumor infiltration; while the remaining 81 % were tumor cell-free (Figure 5B). In patients with positive tumor infiltration, the CSF samples showed a higher absolute number of total cells/µL including T lymphocytes, monocytes and neutrophils compared with CSF samples that were negative for tumor infiltration (Table 1). Notably, FCM was able to detect a minimum number of tumor cells/µ1 (from 0.01/µL). Representative CSF analyses are shown in Figures 6, 7-8.
Table 1 Antibody panel design according to clinical suspicion. Abbreviations: Cy, cytoplasmic antigen; n, nuclear antigen; FITC, fluorescein isothiocyanate; PE, phycoerythrin; PerCP, peridinin-chlorophyll- protein; PECY7, PE cyanin (Cy)7; APC, allophycocyanin; APC7, APC- cyanin (Cy)7; NHL: Non Hodgkin Lymphomas; B-ALL: B cell acute lymphoblastic leukemia; AML: acute myeloblastic leukemia; T-ALL: T cell acute lymphoblastic leukemia; MDS: myelodysplastic syndromes; Others: HIV+ patients, patients with neurological symptoms, autoimmune diseases, etc.


Fig. 6 Identification of B tumor cells in a representative CSF sample from a B-lymphoblastic lymphoma patient. Events enclosed in red identify beads and events enclosed in blue identify total cells. Lymphoma cells (black dots) are positive for CD19, CD20, TdT, Bcl-2 and CD10. Analysis of clonality revealed monoclonal restriction of lambda light chain. Tumor cell count: 5/µL; 88.3 %.

Fig. 7 Illustrative example of a CSF sample from a blast crisis in a patient with chronic myeloid leukemia. For analysis, beads (red dots) and cells (blue dots) are selected. Black dots represent a large population (89.5%) of myeloid blasts with expression of CD45, CD117 and CD38. Blast cell count: 36/µL.
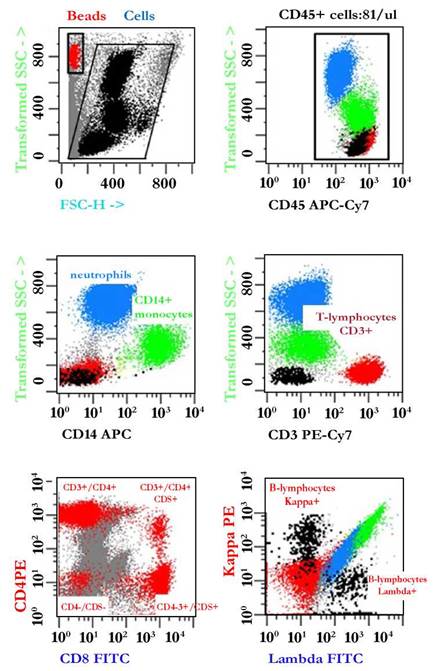
Fig. 8 Illustrative example of the immunophenotypic analysis of leukocyte populations present in normal pleural fluid. For analysis, beads (red dots) and leukocytes (black dots) were selected by gating on Side Scatter -SSC- vs. Forward Scatter -FSC. Next, CD45+ leukocytes were defined by plotting events in a CD45 versus SSC dot plot. Based on differences in their physical parameters, neutrophils (blue dots), monocytes (CD14+ green dots), T lymphocytes (CD3+, CD4+ or CD8+ red dots) and B lymphocytes (black dots) exhibited polyclonal expression of Kappa/Lambda light chains. Total leukocyte count: 81/ µL.
Analysis of pleural fluid samples
Pleural fluid samples (95) were assessed for B-NHL (56 %) tumor infiltration, followed by cases classified as "other," including infectious diseases, solid tumors, cytopenias, HIV+ cases and pleural effusions, representing 21 % of the total pleural fluid samples assayed (Figure 5C). After an analysis of these cases, it was found that 48 (51 %) were infiltrated by tumor cells, while the remaining 47 cases (49 %) were negative for tumor infiltration (Figure 5D). In samples with tumor infiltration, FCM detected a minimum of 0.1 tumor cells/µL. Pleural fluids with tumor infiltration showed a significantly higher absolute number of total cells compared with cases without tumor infiltration. In these samples, varying numbers of neutrophils, monocytes, macrophages, T lymphocytes, B lymphocytes, NK cells, plasma cells, dendritic cells and eosinophils were also detected (Table 2). Examples of pleural fluid analyses with and without tumor infiltration are shown in Figures 9.
Table 2 Normal and tumor cell populations in special samples. NA, not applicable; NS: not significant.


Fig. 9 Example of a pleural fluid sample from a B lymphoma patient with a monoclonal B population with kappa light chain restriction blast. These cells were also positive for CD19, CD45, and CD20. Events encircled in red identify beads and events encircled with blue identify total cells. Tumor cell count: 94/µL; 19%.
Analysis of bronchoalveolar lavage (BAL)
Broncho alveolar lavage samples [24] were studied in order to detect infiltration by B-NHL (46 % of cases) and B-ALL (17 % of cases). Within the "other" category (17 %) were samples from patients with sarcoidosis and lymphadenopathy (Figure 4E). After analysis, tumor infiltration was detected in 21 % of the samples (Figure 4F). For positive cases, FCM detected a minimum of 0.1 tumor cells/µL.
In these samples, varying numbers of neutrophils, monocytes and macrophages, T lymphocytes, B lymphocytes, NK cells, plasma cells, dendritic cells and eosinophils were also determined.
Analysis of other special samples
Pericardial fluid: Eight samples of pericardial fluid were analyzed to assess B-NHL at diagnosis (n = 6), additionally a solid tumor and one case of systemic lupus erythematosus (SLE). All samples were negative for tumor infiltration. T lymphocytes and monocytes were detected in 100 % of the samples. Additionally, neutrophils (7/8 cases), B-lymphocytes (6/8 cases), NK cells (5/8 cases) and plasma cells (4/8 cases) were also detected. The average total cell number was 304/µL (range: 4.5 cells/µL to 762 cells/µL).
Peritoneal fluid: Seven samples of peritoneal fluid were analyzed in patients with multiple myeloma (n = 2), B-NHL (n = 2), carcinoma (n = 1) and anemia (n = 1). Of these, only one case of myeloma was positive for tumor infiltration. An analysis of cellularity revealed that T lymphocytes were detected in 100 % of patient samples, while NK cells and monocytes were detected in 6/7 cases, B-lymphocytes and neutrophils were detected in 5/7 and eosinophils and macrophages were detected in 1/7 cases. The average number of cells detected was 2017/µL with a range of 0.2 cells/µL to 10780 cells/µL. The myeloma sample with tumor infiltration had 2811 pathological plasma cells/µL, which represented 92 % of the total cellularity.
Synovial fluid: One sample was analyzed for the study of MDS during clinical monitoring of the disease. This sample had 2.6 myeloid blasts/µL, which was equivalent to 7.6 % of the total cellularity. T and B lymphocytes and monocytes were also detected. Total cell number was 34 cells/µL.
Ascites: Three samples of ascitic fluid were analyzed, two for the study of B-NHL infiltration and the other for the study of an infectious process. The two cases of B-NHL were negative for tumor infiltration. For all three samples, T lymphocytes, monocytes, B-lymphocytes, NK cells and neutrophils were detected, while plasma cells were detected in two samples. The average total number of cells was 1680/µL with a range of 917 cells/µL to 2659 cells/µL.
Discussion
In recent years, one diagnostic tool that has become very important in clinical practice is FCM immunophenotyping, providing higher sensitivity and efficiency. Therefore, allowing simultaneous analysis of multiple features, compared with other cell analytical techniques. Currently, antibody panels with eight to ten types of fluorescence are used, which give information on cell lineage, maturation stages, aberrant phenotypes and absolute and relative numbers of normal and tumor cells [1-2,6].
Biological sample flow cytometry analysis for the diagnosis and follow-up is very important in routine clinical practice for classification, and monitoring of diseases in conjunction with morphological and molecular analysis [15]. Immunophenotyping has provided relevant information for the diagnosis, classification and monitoring of hematological malignancies and other diseases [2]. Consequently, assessment of immunophenoype by FCM has become essential and is part of the current World Health Organization (WHO) classification of hematological malignancies [16-18].
In 2006 the European Union-supported the EuroFlow Consortium (EU-FP6, LSHB-CT-2006-018708). This project aimed at the prospective design and evaluation of antibody panels for haematological disease diagnosis and classification, where immunophenotyping was proven relevant [17-18]. EuroFlow has contributed in the development of highly sensitive and standardized FCM; particularly in the study of acute leukemias and lymphomas. These protocols describe optimal antibody panels, design and evaluation of adequate standard operating procedures (SOPs) for instrument setup, fluorescence compensation, and sample preparation. Furthermore, elaboration of adequate software tools for overall evaluation of obtained phenotypic profiles [17-18]. These advances have significantly improved minimal residual disease (MRD), detection, assessing therapy response. Moreover, it is considered an important prognostic indicator [18].
Many advantages have been reported for FCM in the study of various types of biological samples such as BM, PB and special samples, including tissue biopsies and body fluids (pleural fluid, pericardial fluid, and CSF, among others, have been reported). Additionally, flow cytometric immunophenotyping has sustained its position as an indispensable tool, as demonstrated by recent studies from the European Consortium EuroFlow. One advantage has been vitreous sample assessment for the study of tumor infiltration by intraocular lymphomas and central nervous system lymphomas [1-2,6]. Therefore, it is vital to ensure sample quality at the pre-analytical phase, to maintain cell viability and cellular antigen integrity [6]. Some samples evaluated by flow cytometry must be processed within 24 hours, in particular BM and PB. However, other samples such as body fluids and biopsies undergo greater damage over time. It has been reported cell death percentages may increase over 80 % between 30 min to 6 h after the sample collection [2,6,13].
In order to obtain better quality samples at the pre-analytical phase, use of commercially available stabilizing solutions has been implemented within the last decade. Examples include Transfix™ (Cytomark, Buckingham, UK) and Cyto-Chex BCT (Streck, Omaha, NE, USA), both validated for clinical use [2,4,13,19-22]. Different studies have reported that PB samples treated with these stabilizers and evaluated at different times post-incubation display increased cell viability and maintenance of cell structures, which provides more reliable results directly reflecting the actual status of the patient [19-21,23-24].
Some studies by different groups have focused on the effect of TransfixTM as a stabilizer evaluation, reporting various technical indications for its use (Table 3) [25].
One of the most important applications of Transfix™ is for absolute CD4+ T cell counts (critical for monitoring HIV+ patients) stability. Additionally, in commercial PB preparations used in quality assurance programs for T cell subpopulation immunophenotyping. These studies have reported that counts are stable at various storage temperatures (4 °C and 25 °C) and for up to 10 d of treatment compared with fresh unstabilized samples [21,24]. However, Transfix™ pre-analysis treatment results in forward- and side-scatter parameter have been shown to change within 24 h to 48 h of addition, as evidenced mainly in neutrophils and monocytes after 10 days of storage at different incubation temperatures [21,26].
Comparative studies on different antigenic marker expression in leukocyte populations between samples with and without TransfixTM are contradictory. Some studies have shown that PB samples without Transfix™ lose antigen expression over time. This phenomenon is associated with apoptotic cell presence as evaluated by propidium iodide staining [26], with significant reduction in absolute counts of leukocyte populations [13]. On the contrary, CD3, CD4, CD38, CD123, and CD45 expression in lymphocytes of patients with primary and secondary immunodeficiencies, leukemias, lymphomas and inflammatory diseases, is stable after incubation with Transfix™. On the other hand, CD45 expression in monocytes decreases significantly with 4 °C incubation from 0 d to 4 d [21,26].
Another parameter evaluated by Canonico B et al. in 2010 [26] was the effect of sample dilution with Transfix™ (1/5 and 1/10 dilutions) in the expression of CD45 in granulocytes and lymphocytes. They showed that CD45 expression was altered by dilutional effects [26]. Moreover, PB cell morphology evaluation by transmission electron microscopy (TEM) evidenced after ten d of storage in Transfix™ and sample incubation at different temperatures (4 °C, 25 °C and 37 °C), lymphocytes retained their morphology compared to other cell types [26].
Other studies have reported Transfix™ use in the study and monitoring of central nervous system infiltration in patients with aggressive lymphomas and acute leukemias. In these studies, Transfix™ was suggested to enhance CSF hematological malignancies detection by preventing cellular loss after 10 d of storage [2,4,7,10,12-13,27].
Although Transfix™ in flow cytometry clinical application has already been reported in various studies, no consensus has been reached on its implementation in clinical practice. Moreover, other studies to evaluate its use in samples other than PB and CSF have not been conducted [28-29]. In the first Colombian Flow Cytometry Consensus of 2008, it was recommended that samples of body fluids be stabilized in case they not processed during the maximum recommended times [6]. It is for this reason that from 2008 onwards, all body fluids samples from patients with different pathologies (both benign and malignant diseases) are collected into tubes containing Transfix™.
Results from the analysis of 1070 samples over a period of 6 y, demonstrate the medical community and patients have significantly benefitted from the implementation of this tool for the study of special samples. These samples are of a greater quality and hence, allow a better classification and staging, with important implications at the therapeutic level for clinical disease monitoring and prognosis. We note that most special samples processed and analyzed corresponded to CSF from patients who were diagnosed with B-ALL. These samples were obtained at different time points during the disease process and have great applicability with other pathologies including other hematological malignancies, solid tumors and other diseases.
Additionally, for all samples tested it is shown that FCM is a tool with great sensitivity. It can detect minute quantities of tumor cells at least 0.01 cells/pL in CSF samples and 0.1 cells/pL in other samples evaluated for cases with tumor infiltration. Flow cytometry can also detect small numbers of normal cells in various body fluids with and without tumor infiltration in a wide range of diseases. It is important to note that in these cases, rare normal cell populations, such as dendritic cells in the pleural fluid, were also detected [1].
For autoimmune diseases, immunodeficiency, infectious diseases and inflammatory processes, FCM is useful for sample cellularity description, as it identifies populations of T lymphocytes, B-lymphocytes, NK cells, plasma cells, eosinophils, basophils, and macrophages, among other cell types [1].
This work represents the first report at the national level supporting TransfixTM implementation in pre-analytical FCM studies of all body fluids that are processed in the clinical practice. In addition, it recommends flow cytometric immunophenotyping as an indispensable tool in our country’s laboratories to guarantee quality results for the benefit of our patients.