Introduction
Coxiella burnetii is a Gram-negative bacterium causing of Q fever, a zoonosis that concerns public health throughout the world. Mammals, birds and arthropods, mainly ticks can be infected, but domestic ruminants (sheep, goats and cattle) are the main reservoirs1, in which C. burnetii is shed through placenta, vaginal discharges, urine, feces and milk2,3. C. burnetii is a frequent cause of reproductive disorders mainly in minor ruminants3-5. In goats, it has been reported as a cause of abortions and stillbirths6,7 and in cows, infection is asymptomatic, but metritis and subclinical mastitis have been described8.
The risk of C. burnetii transmission between animals and from animals to humans depends of the prevalence of shedders and excretion’s levels in ruminants5. C. burnetii is able to persist for long time adverse conditions9 and it is easily transported by the wind10. Inhalation of contaminated dust with the bacterium from products of infected animals is the main source of infection in humans. Nevertheless, infection by consuming raw milk has also been reported11. Clinical spectrum in humans is very broad; patients may experience asymptomatic seroconversion, nonspecific febrile syndrome, atypical pneumonia or hepatitis. Chronic Q fever is rare (<5%), but it occurs in patients with underlying conditions such as immunosuppression, vascular disease, aneurysm, etc., and is mainly expressed with endocarditis that could have a fatal course12.
The epidemiology of Q fever is characterized by a complex interaction of factors like variety of hosts, low infectious dose, nonspecific symptoms, difficult access to diagnostic tools and the lack of epidemiological association, and they provide a masking with others febrile syndromes1. However, in the Q fever epidemic occurred in the Netherlands (2007-2012), more than 4,000 human cases related to farms with infected goats were reported, and it has highlighted its potential impact on human health. The confluence of concentration of infected goat farms near areas with high human density and a favorable meteorological context were among the facilitating factors of bacterial spreading13.
In Colombia, seroprevalence studies of C. burnetii and some human cases of Q fever has been reported 14-16 Additionally, DNA of C. burnetii was detected in 45% of 11 bulk cow milk samples17. Furthermore, in Colombia there is report describing a case of a 56-year-old patient with an associated in agriculture and livestock handling, the diagnose was made using an indirect immunofluorescence assay showed high titers of IgG for C. burnetii anti-phase I (1: 256) and anti-phase II (1:1024)18. However, there is a lack of information of C. burnetii infection in minor ruminants, which are able to transmit the infection. The aim of this study was to provide molecular evidence of C. burnetii infection in sheep and goats from some herds of municipality of Valledupar, Cesar, Colombia.
Materials and Methods
Type of study, geographical area, ruminant’s population, size sample and specimens
A descriptive, prospective and transversal study was performed. Fifteen herds of small ruminants from six villages of municipality of Valledupar, department of Cesar (Colombia) (10º 27´N y 73º 15´O) were chosen at convenience (Figure 1), during March and April 2013. The number of herds and animals per farm were calculated by using of Free-Calc., software19. It was taking to account the population of 20,000 sheep and 8,000 goats and a regional register of 100 herds from Valledupar; a confidence level of 95%, maximum error of 5%, and an expected proportion of infected animals and herds according to previous reports were chosen20. The obtained results were analyzed through descriptive statistic.

Figure 1 Geographical location of goats and sheep herds in the municipality of Valledupar, department of Cesar, Colombia.
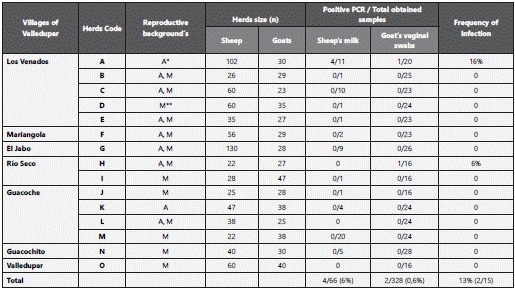
328 female goats and 66 sheep with at least one birth were included in this study. Goats belonged to all 15 herds, whereas sheep came from 11 of them. 15 ml of milk’s ewes and vaginal swabs were collected from goats using sterile cotton swabs. They were placed in sterile plastic tubes, transported to the laboratory at 4°C and subsequently preserved at -20°C. Milk samples showed normal physical characteristics (color, pH and density). The state of C. burnetii infection in herds was unknown at baseline. A herd was considered positive if at least one animal (sheep or goat) was found infected with C. burnetii (milk or vaginal mucus positive by PCR).
DNA extraction and molecular detection of C. burnetii
Milk samples and vaginal swabs were subjected to DNA extraction using the DNA mini kit Purelink (Invitrogen, CA, USA). Milk’s specimens directly from 300 μl of whole homogenized milk were analyzed. 300 μl of TE buffer (10 mM Tris Base, 1 mM EDTA and pH 8) was added to vaginal swabs and mixed by vortex. To ensure no contamination, negative controls (sterile water) were included. DNA was purified in a final volume of 100 μl; according to the manufacturer’s conditions. Sample was stored at -20°C until use as template for Polymerase Chain Reaction (PCR).
A conventional PCR was performed using oligonucleotides CoxP4 (5’-GGCTGCGTGGTGATGG) (Gen bank accession number: M80806) and CoxM9 (GTCCCGGTTCAACAATTCG), previously described with some modifications 21, which amplify a fragment of (435 bp) transposase gene (IS1111) of C. burnetii. All amplified products were visualized in electrophoresis of agarose gel (1.5%); it was purified and sequenced by Macrogen, Korea services. The obtained sequences were edited with MEGA program (version 6.0) and analyzed in BLAST.
Results
Four of 66 (6%) sheep milk and two of 328 (0.6%) vaginal swabs of goats yielded C. burnetii DNA. The transposase gene sequences (IS1111) generated in this study had a percentage of identity of 100 and 99% with C. burnetii strain CbuK Q_154 (Genbank access number CP001020) and C. burnetii strain Guiana Cb175, respectively.
Thirteen percent (n=2) of 15 herds had at least one infected animal (Figure 1). Four milk’s sheep (4/11; 36%) and one vaginal swab from goats (1/16; 5%) were found infected with C. burnetii from a same herd (Table 1). The average of animals in the herds was 77.2 (SD = 33.47, range 1-158). Sheep and goats were mixed in all herds and these latter were predominant. The type of production in sampled herds was 60% (9/15) of ruminants of double purpose (meat and milk production). Farms and its herds were not handled by technical means, and they did not have identification records and reproductive history from animals. However, the occurrence of mastitis, abortions or both were informed in all herds in previous deliveries. Positive herds had records of abortions (Table 1), but the causal agent of such conditions was never identified.
Discussion
The circulation of C. burnetii has been reported in some regions of Colombia. In 2006, a seroprevalence in rural workers from Cordoba and Sucre departments was informed14. A case of endocarditis and one of pneumonia by Q fever were reported in 201215,16, and a case of infection by C. burnetii was reported in a patient with a background in agriculture and livestock handling from a rural area of Monteria, Cordoba18. Furthermore, in a study performed in cattle farms from Monteria, C. burnetii DNA was found in 45% of 11-bulk milk, and 61% of farm workers and residents of the farms had antibodies against C. burnetii17. Herein, we report the infection by C. burnetii in sheep and goats from some herds of Valledupar, Colombia.
The shedding of C. burnetii in sheep and goats is a major issue for public health22. However, studies of frequency of infection in ruminants based on PCR analysis are uncommon23 and this knowledge is important to determine the risk of transmission between animals and from animals to humans5. In the present study, 6% (n=4) of 66 sheep’s milk and 0.6% (n=2) of 328 vaginal swabs from goats yielded C. burnetii DNA. In a study performed in Turkey, out of the 350 bovine milk samples and 250 ovine milk samples collected, 1,42% and 0,4% were found to be positive using the PCR technique, respectively24. The amplification of transposase gene (IS1111)1 allowed for the sensitivity of the assay to be increased, because this a multi-copy gene (7-110 copies)25. DNA sequences generated in the present study confirm that C. burnetii is circulating in goats and sheep from some herds of Valledupar, Colombia.
As it was above described, shedding of C. burnetii can be intermittent, it could increase during postpartum periods, be different between species and vary according to the type of sample. C. burnetii in vaginal mucus of goats is less frequent, but more frequent in milk2. In the present work, this result is in according to the proportion of vaginal mucus samples from goats that yielded C. burnetii DNA (0.6%). Therefore, it is likely that the sample collection in the present study may have coincided with the shedding period for some individuals and not with others. Additionally, only one type of sample was obtained from each ruminant species (ovine milk and mucus vaginal from goats), and taken together these results, we suggest that a concomitant analysis of various and different types of samples from the same animal might increase the probability of finding an infected animal with C. burnetii. Moreover, Guatteo et al.,5 reported that infected animals shed C. burnetii mainly during parturition; in our study, samples were collected several months after delivery in herds. However, in a study carried out by Rodolakis et al.,2, it was found that shedding of C. burnetii could not be related with the parturition. C. burnetii DNA was found in samples of milk, mucus vaginal and feces taken from 0 to 421 days after parturition in bovine herds, from 5 to 119 days in caprine and 11 to 238 days in ovine herds. The results of this study might suggest that the excretion of C. burnetii could be higher in the studied animals and could vary in different times. These results also suggest that the excretion of C. burnetii in infected animals could create a public health risk for people in the immediate surroundings as well as in surrounding areas. Additional studies are necessary, with a larger number of animals and with several samples in different periods. Likewise, it is important to carry out studies on other species, such as rodents and ticks, which have been described to be included in the epidemiological cycle of C. burnetii26.
In this study, 13% (n=2) of 15 herds had at least one infected animal with C. burnetii. One herd had five infected animals, four sheep’s shed C. burnetii in milk and one goat shed in vaginal mucus, and it showed that this herd had active circulation of C. burnetii. In a study performed in Germany, C. burnetii DNA was found in 5% of 39 flock’s sheep27. In the Netherlands, C. burnetii DNA was detected in 33% of 292 goat farms28. In Italy, C. burnetii DNA was amplified in 18% of 199 goats and sheep farms with reproductive history29. In the present study, all herd owners reported the occurrence of reproductive problems (abortions and/or mastitis) in previous deliveries, but causal agent of such conditions were undetermined. One of positive herds to C. burnetii have a history of abortions and other one have history of abortions and mastitis, however, others studies are necessary to determine causal agent of this disorders.
On the other hand, Q fever is an important zoonosis. However, In Colombia and Latin American countries, Q fever is a neglected disease due to great multiplicity of symptoms, absence of knowledge of the disease and epidemiological data, which most expected lead to underdiagnoses and underreporting of the disease. This is the first report in the Caribbean area of Colombia, which the main reservoirs and sources of human infections were above described, however, ticks also are involved and the disease presents with a variety of clinical manifestations as atypical pneumonia, febrile hepatitis and endocarditis may also occur. The variability in the clinical manifestations of Q fever may lead to postponement of diagnosis. Therefore, anamnesis, epidemiological factors and serological tests are tremendously important in Colombia. Being exposed to livestock, living in rural area or living closely to farms are public health risk factors. Above that, the lack of direct contact with animals cannot disregard the diagnosis of Q fever, since airborne transmission of C. burnetii is also recurrent.
Conclusion
We report the C. burnetii infection in sheep and goats from some herds in Valledupar, Colombia. Due to environmental stability and potential aerosol dispersion of C. burnetii, our findings highlight the possibility of occurrence of infections in humans and animals in Valledupar, Colombia. The detection of C. burnetii in sheep milk could represent a public health risk factor for people who consuming frequently raw milk, cheeses or other products. Further studies are necessary to evaluate other routes such as tick’s bite, feces, milk from goats and vaginal mucus from sheep of this region of Colombia. Clinical differential diagnoses including Q fever in high-risk people should be taken into account in Colombia.