Remark
| 1) Why was this study conducted? |
| To evaluate the CRT implementation in a large series of real-life patients with AHF and LBBB. |
| 2) What were the most relevant results of the study? |
| CRT implementation was delayed and underused in patients with AHF and LBBB. Under these circumstances, CRT was not associated with a reduction in all-cause mortality in the long term. |
| 3) What do these results contribute? |
| These results make us reflect on the late diagnosis of refractory heart failure, and the underuse of CRT implementation, strongly recommended in the guidelines. |
Introduction
Heart failure (HF) is a prevalent clinical syndrome with high morbidity and mortality rates. The prognosis of patients with HF has improved considerably in the last decades, but it remains poor, and the improvement in prognosis has been confined to those with reduced ejection fraction (HFrEF) in approximately 50% of all patients with HF 1. Cardiac resynchronization therapy (CRT) was approved in 2005 to treat patients with refractory HF. In appropriately selected individuals, CRT improves cardiac function, enhances quality of life, and reduces morbidity and mortality 1,2. Nonetheless, assessing the response to CRT is challenging and different studies have shown that patients with left bundle-branch block (LBBB) morphology are more likely to respond favourably to CRT. Current guidelines recommend the use of CRT in symptomatic patients with HF in sinus rhythm with a QRS duration ≥150 ms and LBBB QRS morphology and with a left-ventricle ejection fraction (LVEF) ≤35% despite optimal medical treatment to improve symptoms and reduce morbidity and mortality 2,3. The question of the timing of CRT is controversial, and as the efficacy of the medical treatment can be limited in patients with LBBB, earlier CRT implementation has been suggested 3. Nevertheless, despite being one of the most effective therapies to treat the symptoms of HFrEF, up to two-thirds of eligible patients are not referred for CRT, and the causes of disregarding CRT have not been extensively investigated. Moreover, there is considerable heterogeneity in CRT use among the different European countries, with Italy having the highest implantation rates (followed by Denmark and the Czech Republic) and Ukraine the lowest 4,5.
The present study aimed to determine the indication and timing for CRT and its impact on prognosis in patients with HF and LBBB from a real-live registry: the Spanish EAHFE (Epidemiology of Acute Heart Failure in Emergency Departments) cohort.
Materials and Methods
Study population and patient selection
The present study was a secondary analysis of patients included in the EAHFE registry, the design of which has been explained in greater detail elsewhere 6. Briefly, the EAHFE cohort is a prospective multicentre registry that includes patients with acute HF (AHF) attended in 45 Spanish Emergency Departments (EDs) independently of their final disposition after the first medical presentation (admission to a general ward, admission to intensive care unit or discharged home). Follow-up visits are mandatory at 90 and 365 days after hospital discharge; subsequently, the vital status of the patients is reviewed annually. The EAHFE cohort design was approved by the Ethics Committees of all the participating hospitals. The study was performed following the ethical standards of the Declaration of Helsinki and written informed consent was obtained from all participating patients. For the present secondary analysis, we included patients from the EAHFE registry with LBBB, whose information on the electrocardiogram (ECG) at baseline and vital status were available. Patients with pacemakers or CRT at the time of inclusion in the registry were excluded from this analysis.
Classificatory variables
Patients with LBBB were classified according to whether they were treated or not with CRT. This, included patients receiving CRT after compensation for the index AHF episode and patients with CRT implantation at some point during their follow-up. Those who were not treated with CRT at any time during follow-up were classified according to whether they had or did not have criteria for CRT. Criteria for patients with LBBB to be treated with CRT implantation were to have a LVEF < 35% for CRT and receive optimal medical treatment.
Independent variables
We included age and sex, variables corresponding to patient baseline status (Barthel index, New York Heart Association [NYHA] and LVEF), 15 comorbidities (smoking, hypertension, dyslipidaemia, diabetes mellitus, coronary artery disease, heart valve disease, peripheral artery disease, cerebrovascular disease, atrial fibrillation, chronic HF, chronic kidney disease, chronic obstructive pulmonary disease, dementia, active neoplasia and liver cirrhosis) and baseline treatments.
Endpoints
The primary outcome was all-cause mortality during ten years of follow-up. Time was considered from the day of hospital discharge after treatment and stabilization of the decompensation. Patients dying during the index AHF episode before discharge (in-hospital mortality) were not included in the analysis. Outcome adjudication was carried out at a local level by the principal investigators of each center. For this purpose, local investigators contacted patients or relatives by phone, reviewed patients’ medical reports or consulted the national healthcare registry (as the public healthcare system covers more than 99% of the Spanish population) to check for patient death.
Statistical analysis
Quantitative variables are expressed as median and interquartile range (IQR). Qualitative variables are expressed as the number of patients and percentages. The chi-square or Fisher exact tests (as needed) were used to compare the distribution of qualitative variables in patients with and without CRT. The non-parametric Mann-Whitney U test was used to compare quantitative variables. Ten-year all-cause mortality for the whole cohort was plotted using the Kaplan-Meier method. The reverse Kaplan-Meier method was used to calculate the median follow-up time in the cohort. A multivariable Cox proportional hazards model was used to evaluate the association of CRT with 10-year all-cause death. To account for indication bias, we adjusted for known reasons for treatment selection (i.e., known reasons for indicating CRT), which resulted in conditioning for age, sex, optimal medical therapy, baseline cardiac rhythm, LVEF and Barthel index. To avoid immortal time bias (i.e., patients in the CRT group could not have died until receiving the CRT. Hence there is an interval during which the outcome event could not have occurred), we modelled CRT as a time-dependent covariate by building a time-dependent set with the tmerge function of the survival package of R software 7. In addition to reporting the hazard ratio (HR) for patients receiving CRT, a conditional effects plot was constructed to represent the survival probability of an “average” subject who either did or did not receive CRT 8. As a sensitivity analysis, we calculated the adjusted HR for patients receiving CRT using as the comparator only those patients who did not receive CRT but had criteria for CRT implementation.
Statistical significance was accepted if the p-value was <0.05 or if the 95% confidence interval (CI) excluded the value 1. All the statistical analyses were performed with the Statistical Package for Social Sciences version 23.0 (IBM, Armonk, NY, USA) and the R Foundation for Statistical Computing (version 4.1.2).
Results
The present study included 729 patients with AHF and LBBB who had not previously been treated with a pacemaker or CRT (Figure 1). The baseline characteristics are presented in detail in Table 1. Briefly, patients with AHF and LBBB from the EAHFE registry had advanced age (median age 82 years), a high burden of comorbidities (the most frequent being hypertension (84%), chronic HF (66%), dyslipidaemia (46%), diabetes mellitus (45%) and atrial fibrillation (43%) and some degree of functional dependence (measured with the Barthel index). The median [IQR] was 40 [25] and the predominant category, according to LVEF, was HFrEF, which comprised 46% of patients.
Table 1 Baseline characteristics and differences according to cardiac resynchronization therapy.
| AHF with LBBB N= 729 | Missing N (%) | CRT N= 46 | No CRT (all patients) N= 683 | P* | No CRT (only patients fulfilling criteria for CRT) N= 108 | p* | |
|---|---|---|---|---|---|---|---|
| Demographic data | |||||||
| Age (years) - median [IQR] | 82 [13] | 0 | 66.9 [16.1] | 82.8 [11.9] | *** | 81.4 [11.9] | *** |
| Sex female | 381 (52.3%) | 0 | 20 (43.5%) | 361 (52.9) | - | 46 (42.6%) | - |
| BMI (kg/m2) - median [IQR] | 27.6 [5.8] | 309 (42) | 29.2 [20.3] | 27.4 [6.0] | - | 27.7 [5.9] | - |
| Comorbidity | |||||||
| Active smoker | 47 (6.4%) | 169 (23.2) | 5 (13.5%) | 42 (8.0%) | * | 9 (10.2%) | - |
| Hypertension | 612 (84.0%) | 1 (0.1) | 33 (71.7%) | 579 (84.9%) | * | 92 (85.2%) | - |
| Dyslipidaemia | 333 (45.7%) | 2 (0.3) | 28 (60.9%) | 305 (44.8%) | * | 50 (46.3%) | - |
| Diabetes mellitus | 325 (44.6%) | 1 (0.1) | 23 (50.0%) | 302 (44.3%) | - | 62 (57.4%) | - |
| Coronary artery disease | 233 (32.0%) | 1 (0.1) | 10 (21.7%) | 223 (32.7%) | - | 58 (53.7%) | *** |
| Heart valve disease | 211 (28.9%) | 1 (0.1) | 14 (30.4%) | 197 (28.9%) | - | 41 (38.0%) | 0.372 |
| Peripheral arterial disease | 68 (9.3%) | 1 (0.1) | 4 (8.7%) | 64 (9.4%) | - | 8 (7.4%) | 0.785 |
| Cerebrovascular disease | 89 (12.2%) | 1 (0.1) | 4 (8.7%) | 85 (12.5%) | - | 11 (10.2%) | 0.775 |
| Atrial fibrillation | 312 (42.8%) | 1 (0.1) | 10 (21.7%) | 302 (44.3%) | ** | 45 (41.7%) | * |
| Chronic heart failure | 478 (65.6%) | 24 (3.3) | 27 (60.0%) | 451 (68.3%) | - | 90 (83.3%) | ** |
| Chronic kidney disease | 198 (27.2%) | 1 (0.1) | 12 (26.1%) | 186 (27.3%) | - | 38 (35.2%) | 0.270 |
| COPD | 152 (20.9%) | 2 (0.3) | 9 (19.6%) | 143 (21.0%) | - | 18 (16.8%) | - |
| Dementia | 69 (9.3%) | 54 (7.4) | 0 (0%) | 68 (10.8%) | * | 9 (9.8%) | * |
| Active cancer | 88 (12.1%) | 54 (7.4) | 8 (18.6%) | 80 (12.7%) | - | 14 (15.2%) | - |
| Cirrhosis | 6 (0.8%) | 54 (7.4) | 0 (0%) | 6 (0.9%) | - | 0 (0%) | - |
| Baseline status | |||||||
| NYHA class | 37 (5.1) | ||||||
| I | 170 (23.3%) | 7 (16.7%) | 163 (25.1%) | - | 17 (16.8%) | ||
| II | 373 (51.2%) | 31 (73.8%) | 342 (52.6%) | 57 (56.4%) | - | ||
| III | 147 (20.2%) | 4 (9.5%) | 143 (22.0%) | 26 (25.7%) | |||
| IV | 2 (0.3%) | 0 (0%) | 2 (0.3%) | 1 (1.0%) | |||
| LVEF (%) - median [IQR] | 40 [25] | 76 (10.4) | 35 [24] | 40 [25] | * | 30 [10] | *** |
| Preserved LVEF (≥50%) | 252 (34.6%) | 12 (21.6%) | 240 (39.5%) | - | 0 (0%) | *** | |
| Mildly Reduced LVEF (41-49%) | 64 (8.8%) | 3 (6.5%) | 61 (10.0%) | 0 (0%) | |||
| Reduced LVEF (≤40%) | 337 (46.2%) | 31 (67.4%) | 306 (50.4%) | 108 (100%) | |||
| Barthel index (points) - median [IQR] | 90 [30] | 68 (9.3) | 100 [0] | 90 [30] | *** | 95 [23] | *** |
| Chronic treatments at home | |||||||
| Loop diuretics | 477 (65.4%) | 11 (1.5) | 19 (41.3%) | 458 (68.2%) | *** | 83 (76.9%) | *** |
| RAASI | 463 (63.5%) | 11 (1.5) | 35 (71.6%) | 428 (63.7%) | - | 108 (100%) | *** |
| Beta-blockers | 369 (50.6%) | 10 (1.4) | 23 (50.0%) | 346 (51.4%) | - | 108 (100%) | *** |
| MRA | 139 (19.1%) | 10 (1.4) | 11 (23.9%) | 128 (19.0%) | - | 26 (24.1%) | - |
| Optimal treatment 1 (RAASI +BB) | 256 (35.1%) | 11 (1.5) | 21 (45.7%) | 235 (35.0%) | - | 108 (100%) | *** |
| Optimal treatment 2 (RAASI+BB+MRA) | 56 (7.7%) | 11 (1.5) | 8 (17.4%) | 48 (7.1%) | * | 26 (24.1%) | - |
BMI: body mass index; COPD: chronic pulmonary obstructive disease; CRT: cardiac resynchronization therapy; IQR: interquartile range; LBBB: left bundle branch block; LVEF: left ventricular ejection fraction; MRA: mineral corticosteroid-receptor blockers; NYHA: New York Heart Association; RAASI: renin-angiotensin-aldosterone system inhibitors (includes angiotensin-converting enzyme inhibitors, angiotensin receptor antagonists or angiotensin receptor-neprilysin inhibitors); BB: beta-blockers
p >0.05; * p <0.05; ** p <0.05; *** p <0.001
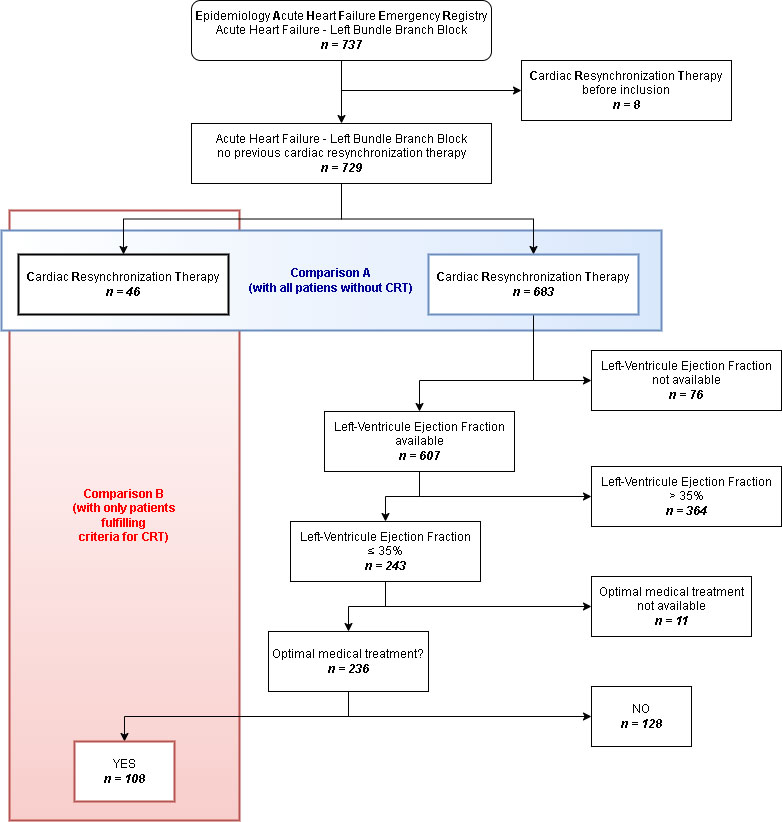
Figure 1 Flowchart for patient inclusion and analysis. *Optimal medical treatment includes renin-angiotensin-aldosterone system inhibitors and beta blockers. Abbreviations: AHF: acute heart failure; CRT: cardiac resynchronization therapy; LBBB: left bundle-branch block; LVEF: left-ventricle ejection fraction
There were 46 patients (6.3%) treated with CRT at some point in the follow-up, with a median [IQR] time for CRT implementation of 960 [1,147] days. When comparing treated and untreated patients (Table 1), patients treated with CRT were younger, had different comorbidities (less hypertension, atrial fibrillation and dementia and more dyslipidaemia and smoking habit), less functional dependence (higher Barthel index values) and lower LVEF values. The only difference found according to treatment was that patients from the CRT group were less frequently treated with loop diuretics.
In addition to the 46 patients treated with CRT, there were 108 patients with LVEF less than or equal to 35%, on optimal treatment (fulfilling criteria for CRT according to guidelines) at their initial evaluation for inclusion in the EAHFE cohort and had not received CRT at any point during follow-up. This means that the device was finally implanted in only 3 in 10 patients (46 out of 154), fulfilling the criteria for CRT. Characteristics associated with not receiving CRT in this subgroup were similar to those observed for the whole group of patients with LBBB not treated with CRT (Table 1).
Outcome
The median follow-up was 5.7 years (95% CI: 5.6-5.8). Kaplan-Meier survival curves for 10-year all-cause mortality stratified according to CRT are presented in Figure 2A and survival probability curves for an average patient of our cohort in the CRT or no CRT group are presented in Figure 2B.
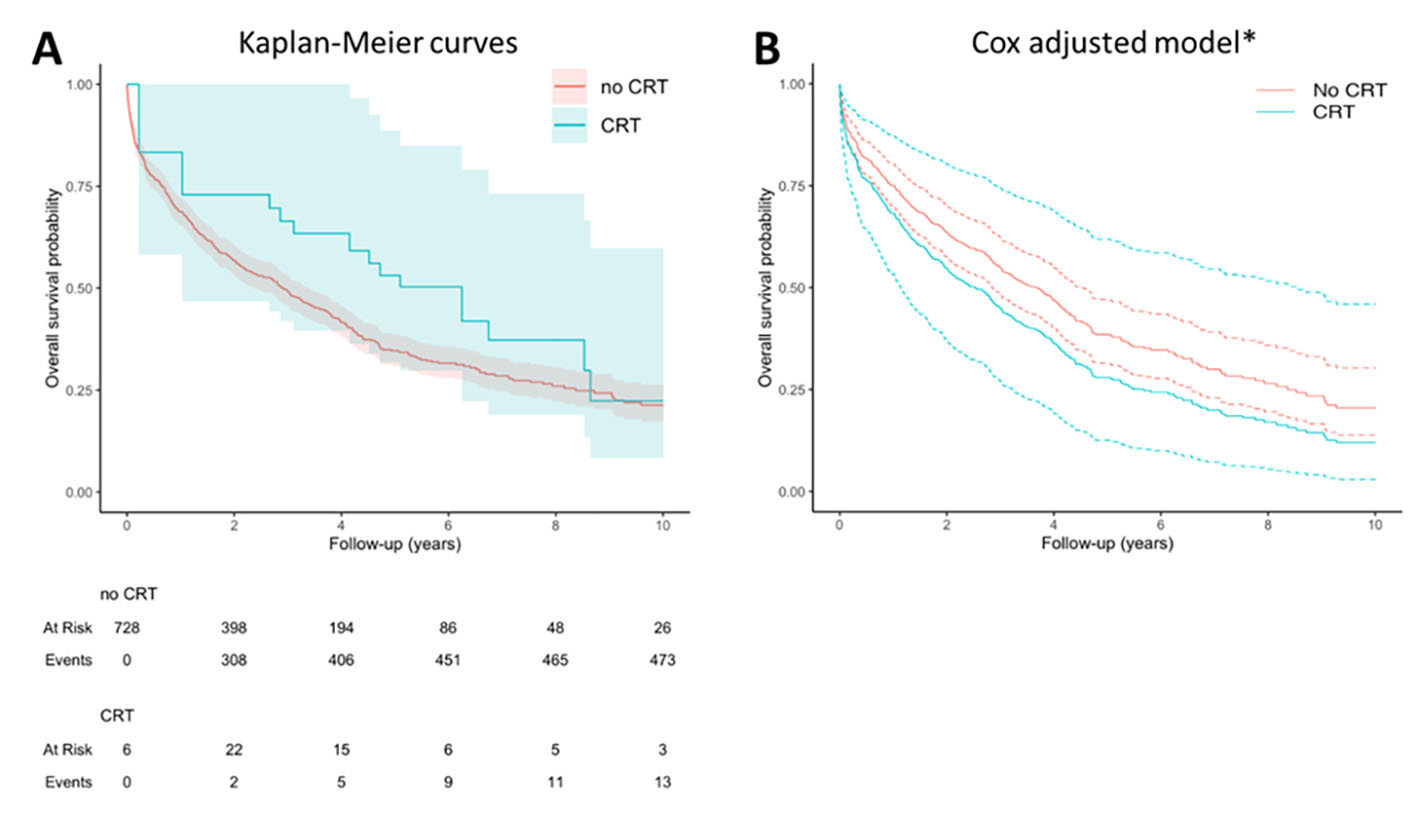
Figure 2 Analysis of survival. Panel A shows the Kaplan-Meier curves comparing the survival between 2 groups: CRT (46 patients) vs no CRT (683 patients). CRT is modelled as a time dependent covariate to avoid the immortal time bias due to the fact that patients in the CRT group could not have died until receiving the CRT. Hence, there is an interval during which the outcome event could not have occurred. For this reason, despite 46 patients being included in the CRT group, the number of patients at risk in this group at time zero is 6 instead of 46, and then the number of patients at risk increases (as they are treated with RCT) or decreases (as they die or are censored) during follow-up. Panel B shows the survival probability obtained in the adjusted Cox model with conditional effects of an “average” subject which either received or did not receive CRT.*Conditioned to median age (82), female sex, no atrial fibrillation, not on optimal medical therapy, left ventricular ejection fraction of 40% and a Barthel index of 90 points. Abbreviations: CRT: cardiac resynchronization therapy.
When comparing patients with and without CRT, there was a lack of evidence supporting CRT as a beneficial therapy for reducing 10-year mortality (adjusted HR 1.33, 95% CI: 0.72-2.48; p: 0.4). Sensitivity analysis using only untreated patients fulfilling CRT criteria showed similar results (adjusted HR 1.34, 95% CI: 0.67-2.68).
Discussion
In this large series of real-life patients with AHF and LBBB we found that after a long-term follow-up, CRT was only applied in 46 (6%) patients, with CRT being implemented after a median time of nearly three years. The remaining 683 patients were not treated with CRT and, in those for whom information on the LVEF was available, 108 would have been potential candidates for CRT according to guidelines recommendations (LVEF ≤35% and optimal medical treatment). Therefore, at least 154 out of 729 patients (21%) were candidates to receive CRT at the time of inclusion in the EAHFE cohort, although only 3 in 10 were finally treated despite the long follow-up performed in the present study. Other studies have reported percentages of eligible patients ranging between 5 and 10% 9) but these studies included unselected patients hospitalized with HF and not only patients with LBBB.
Advanced age, comorbidities, and functional status could explain, in part, the late and scarce implementation of CRT. Other factors that may have contributed to this very delayed and low implementation of CRT are preserved or mildly-reduced LVEF (43%) or mildly symptomatic patients (NYHA functional class II in 51% of patients) at the time of inclusion, a low percentage of optimal medical treatment for HFrEF and/or the presence of atrial fibrillation at baseline (43%).
The presence of LBBB in the context of HF constitutes a marker of a more evolved cardiac disease and it is associated with an increased risk of all-cause mortality 3,10. It is therefore important, especially when the LVEF is reduced, to carefully assess the indication of CRT to improve the prognosis. Because of the results and consistent with other studies, it seems that the use of CRT is highly delayed and underused 4,5,9 and there is room for improvement to increase its use, as clinical trials have demonstrated that CRT is associated with an improvement in patient symptoms, functional capacity and survival.
In the present study, treatment with CRT was not associated with improving long-term prognosis despite adjusting the analysis for possible confounding factors and the selection and immortal time biases. The high risk of death at ten years of follow-up in advanced-age patients with comorbidities after an episode of AHF (survival was less than 25%), along with the delay in implementing CRT are likely to outweigh the benefits of implementing CRT in this population. Furthermore, it is important to note that CRT studies were mostly performed 20 years ago and thereby prior to the widespread use of current HF therapies, and medical therapy may well have improved substantially, so that even in this specific phenotype, this expensive, invasive intervention may no longer provide improved mortality. However, it may still provide improved quality of life.
Some limitations in this study should be considered. First, although we accounted for confounding by indication bias by adjusting for known reasons for CRT treatment indication (e.g., sinus rhythm, LVEF and optimal medical therapy), we were unable to adjust for all the known reasons (e.g. QRS duration) as they were not recorded in our data set. As a result, comparisons of risks for an outcome between exposed (CRT) and unexposed (no CRT) subjects would be biased toward a higher risk among the exposed (CRT), explaining (in part) why we observed a lack of evidence for benefits in 10-year mortality for CRT. Second, patient classification was based on LVEF values at inclusion, which was missing in 10% of cases. In addition, LVEF is a dynamic value that can change throughout the evolution and natural history of HF (improving in some cases and worsening in others) and some patients can be reclassified according to the new LVEF values. In the present study, we did not have this information, which could have modified the criteria for implementing CRT.











 text in
text in 



