Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Avances en Psicología Latinoamericana
Print version ISSN 1794-4724
Av. Psicol. Latinoam. vol.31 no.1 Bogotá Jan./Apr. 2013
Corticosterone plasma concentrations in Carioca High-and Low-conditioned freezing rats after a fear conditioned task
Concentraciones plasmáticas de corticosterona en ratas Carioca Alto y Carioca bajo congelamiento condicionado luego de una tarea de miedo condicionado
Concentrações plasmáticas de corticosterone em ratos Carioca Alto- e Carioca Bajo-Congelamento condicionado após uma tarefa de medo condicionado
LAURA ANDREA LEÓN A.*
VITOR CASTRO GOMES**
MARCUS LIRA BRANDÃO***
CELSO RODRIGUES****
FERNANDO P. CARDENAS*****
J. LANDEIRA-FERNANDEZ******
* Psychologist and Master of Science in Psychology - Universidad de los Andes, Bogotá, Colombia. cPhD in Psychobiology - Universidade de São Paulo, Ribeirão Preto, Brasil. Departamento de Psicologia, Faculdade de Filosofia Ciências e Letras de Ribeirão Preto, Universidade de São Paulo, Ribeirão Preto, Brazil. Departamento de Psicologia, Faculdade de Psicologia, Pontifícia Universidade Católica de Rio de Janeiro,
e-mail: laurandrea@gmail.com, +55(16)36023788.
** Psychologist, Master of Sciences and PhD - Pontifícia Universidade Católica de Rio de Janeiro. Departamento de Psicologia, Faculdade de Psicologia, Pontifícia Universidade Católica de Rio de Janeiro, Rio de Janeiro, Brazil.
*** Physician - Universidade Federal de Espírito Santo, Master of Sciences and PhD in Pharmacology - Universidade de São Paulo. Departamento de Psicologia, Faculdade de Filosofia Ciências e Letras de Ribeirão Preto, Universidade de São Paulo, Ribeirão Preto, Brazil.
**** Master of Sciences and PhD in Physiology - Universidade de São Paulo. Departamento de Fisiologia, Faculdade de Medicina de Ribeirão Preto, Universidade de São Paulo, Ribeirão Preto, Brazil.
***** Psychologist - Universidad Nacional de Colombia, Master of Sciences and Phd - Universidade de São Paulo, Ribeirão Preto, Brazil. Chair of the Laboratory of Neuroscience and Behavior, Department of Psychology, Facultad de Ciencias Sociales, Universidad de los Andes, Bogotá, Colombia. Laboratory of Neuroscience and Behavior, Department of Psychology, Universidad de los Andes.
****** Psychologist - Pontifícia Universidade Católica de Rio de Janeiro, Master of Sciences in Experimental Psychology - Universidade de São Paulo, PhD in Behavioral neuroscience - University of California at los Angeles. Departamento de Psicologia, Faculdade de Psicologia, Pontifícia Universidade Católica de Rio de Janeiro, Rio de Janeiro, Brazil. Curso de Psicologia, Universidade Estácio de Sá.
Para citar este artículo: León, L. A., Gomes, V. C, Brandão, M. L., Rodrigues, C., Cárdenas, F. P. & Fernandez, J. L. (2013). Corticosterone plasma concentrations in Carioca High- and Low-conditioned freezing rats after a fear conditioned task. Avances en Psicología Latinoamericana, 31 (1), pp. 279-287.
Fecha de recepción: 1° de agosto de 2012
Fecha de aceptación: 22 de octubre de 2012
Abstract
Our group in the Psychology Department at Pontifícia Universidade Católica do Rio de Janeiro (PUC-Rio) developed a rat genetic model of extreme freezing in response to contextual cues in an experimental chamber previously associated with footshock. One of the lines, Carioca High Freezing (CHF), exhibits an enhanced conditioned freezing response, whereas the other line, Carioca Low Freezing (CLF), shows the opposite response. The present study investigated corticosterone concentration between these two lines of animals and a random (RND) line of rats both under basal conditions and test condition after an emotional challenge using a contextual fear conditioning protocol. Comparisons between basal and test plasma corticosterone concentrations suggested differential basal and fear-induced differences between the two lines. The differences between basal conditions is an important and relevant aspect to be considered in behavioral experiments using or assessing stress and could help to understand variability in naïve populations.
Keywords: anxiety traits, corticosterone, fear, rats
Resumen
Casi toda la investigación farmacológica en estrés y desórdenes de ansiedad es realizada generalmente en poblaciones de animales que se supone son comparables. En el laboratorio de Neurociencia Comportamental de la Pontificia Universidad Católica de Río de Janeiro (Brasil), dos nuevas líneas de ratas Wistar fueron aisladas por la selección fenotípica de la respuesta emocional en el protocolo de condicionamiento de la respuesta de miedo. Una de las líneas, denominada Carioca High Freezing (CHF) muestra una respuesta aumentada de congelamiento en el test, mientras que la otra -Carioca Low Freezing (CLF)- muestra la respuesta opuesta. Aquí presentamos datos orientados a evaluar las condiciones basales y la vulnerabilidad al estrés entre las dos líneas. Los niveles de corticosterona fueron comparados entre las dos líneas tanto en condiciones basales como después de un desafío emocional utilizando el protocolo de miedo condicionado. La comparación entre las concentraciones plasmáticas de corticosterona basal y luego del retest sugiere diferencias basales y diferencias inducidas por el miedo entre las dos líneas. Las diferencias en las condiciones basales es un aspecto importante y relevante que debe ser considerado en experimentos comportamentales que usen o evalúen el estrés y podría ayudar a comprender la variabilidad encontrada en las poblaciones.
Palabras clave: rasgos de ansiedad, corticosterona, miedo, ratas
Resumo
Nosso grupo no Departamento de Psicologia da Universidade Católica do Rio de Janeiro desenvolveu um modelo genético com ratos que apresentam respostas extremas de congelamento a estímulos contextuais de uma caixa experimental previamente associados a choques elétricos. Uma das linhagens, Carioca High Freezing (CHF) apresenta uma resposta aumentada de congelamento condicionado. A outra linhagem, Carioca Low Freezing (CLF) apresenta a resposta de congelamento condicionada em direção oposta. O presente trabalho comparou os níveis de corticosterona entre as duas linhagens assim como uma terceira linhagem com cruzamento aleatório (RND) tanto em condições basais assim como em um teste onde os animais foram expostos á situação de condicionamento contextual de medo. A comparação entre as concentrações plasmáticas de corticosterona basal e depois do teste sugere diferenças basais assim como diferenças induzidas pelo medo condicionado entre as duas linhagens. As diferenças nas condições basais é um aspecto importante e relevante que deve ser levado em consideração em experimentos que avaliem o estresse e pode ajudar na compreensão da variabilidade nas populações.
Palavras chave: traço de ansiedade, corticosterona, medo, ratos
Introduction
Extensive scientific research has been conducted to understand the psychobiological components of stress and related psychopathologies, such as anxiety disorders. Stress activates physiological and behavioral responses to prepare the animal to cope with new and challenging situations (Sandi, 2004). When aversive stimuli become chronic, the resultant sustained activation of neuroendocrine, immune, and cardiovascular systems leads to enhanced vulnerability to develop psychopathologies and cognitive impairments (Aisa, Tordera, Lasheras, Del Rio, & Ramirez, 2007; Bisaz, Schachner, & Sandi, 2011; Blazevic, Colic, Culig, & Hranilovic, 2012; Sandi, & Richter-Levin, 2009). Based on Blanchard and Blanchard's work, McNaughton, & Corr (2004) proposed a functional hierarchy that determines the appropriate behavior in relation to defensive distance. Shorter defensive distances activate more caudal (subcortical) structures to produce active responses (i.e., panic-like behavior, such as fight or flight) related to panic attacks. Longer defensive distances activate more rostral (cortical) structures to produce behavioral inhibition (i.e., freezing), reactions that may be related to General Anxiety Disorder (GAD). Generalized Anxiety Disorder is a very common stress-related pathology characterized by excessive concern about everyday events that affects the normal functioning of the individual in all aspects of life (Barlow, Blanchard, Vermilyea, Vermilyea, & DiNardo, 1986; Graeff, & Zangrossi, 2010).
The majority of behavioral pharmacological research is performed in heterogeneous populations of animals while not considering possible individual differences or the particular history of a specific subject. Human and animal studies show that individual differences affect the way that individuals cope with environmental challenges (Bardi et al., 2012; Blanchard, Hynd, Minke, Minemoto, & Blanchard, 2001; Gomez-Lazaro, Garmendia, Beitia, Perez-Tejada, Azpiroz, & Arregi, 2012; Metna-Laurent et al., 2012; Oitzl, Champagne, van der Veen, & de Kloet, 2010). Some individuals within the same population have been shown to display higher vulnerability to stress, whereas others appear to be resilient to developing stress-induced psychopathological symptoms (Aisa, Tordera, Lasheras, Del Rio, & Ramirez, 2008; Bolger, & Zuckerman, 1995; Hammen, 2005; Oitzl et al., 2010; Sandi, & Richter-Levin, 2009; Uchida et al., 2008). From this perspective, we can assume that the environment and some genetically determined predisposing factors, in addition to the neural circuitry responsible for emotional defensive reactions, play a significant role in the pathogenesis of stress-related disorders. Our group in the Psychology Department at Pontifícia Universidade Católica do Rio de Janeiro (PUC-Rio) developed a rat genetic model of extreme freezing in response to contextual cues in an experimental chamber where animals were exposed to three unsignaled electric footshocks on the previous day. The line selected for high conditioned freezing is known as Carioca High Freezing (CHF), and the line selected for low conditioned freezing is known as Carioca Low Freezing (CLF). A random (RND) line of randomly selected rats was also used as a control group for the CHF and CLF lines (Gomes & Landeira-Fernandez, 2008).
Physiological responses to stressful situations are mediated by autonomic nervous system activity and the hypothalamic-pituitary-adrenal (HPA) axis. The HPA axis is part of the neuroendocrine system that controls reactions to acute and chronic stress (Castro et al., 2012; Harris, & Seckl, 2011; Raber, Akana, Bhatnagar, Dallman, Wong, & Mucke, 2000; Sandi, Cambronero, Borrell, & Guaza, 1992). Many human studies have shown that anxiety-related stimuli activate the HPA axis and increase cortisol levels (Abelson, Khan, Liberzon, & Young, 2007). The same activation could be observed in rats previously exposed to aversive situations, such as maternal separation, social isolation, and the elevated plus maze. In these situations, animals exhibit an increase in plasma corticosterone concentrations (Aisa et al., 2007; Reis, Albrechet-Souza, Franci, & Brandão, 2012). Based on these findings, the Carioca lines developed by our group may be hypothesized to present differences in plasma corticosterone levels after an emotional challenge. Therefore, the purpose of the present study was to quantify plasma corticosterone concentrations under both basal and stressful conditions in the CHF and CLF lines and RND control rats.
Materials and methods
Animals
Rats from the CHF (n = 7), CLF (n = 7), and RND (n = 7) lines from the 11th generation were used in the present study. The animals were selected according to the same procedure described by Gomes, and Landeira-Fernandez (2008). All of the animals were housed in groups of four in polycarbonate cages (18 ' 31 ' 38 cm) with food and water available ad libitum. Three days before any experimental procedure, all of the animals were handled for 2 min per day according to the special handling procedures reported by Fluttert, Dalm, and Oitzl (2000) for sequential blood sampling by tail incision. All of the experiments reported in this article were performed in accordance with the recommendations ofthe Brazilian Society of Neuroscience and Behavior and complied with the United States National Institutes of Health Guide for Care and Use of Laboratory Animals. The procedures were approved by the Committee for Animal Care and Use, Pontifícia Universidade Católica de Rio de Janeiro, Brazil.
Apparatus
Contextual fear conditioning occurred in four observation chambers (25 ' 20 ' 20 cm), each placed inside a sound-attenuating box. Each chamber was illuminated with a red light (25 W). A 78 dB noise was supplied by a white noise generator. The chamber had a grid floor (15 stainless steel rods spaced 1.5 cm apart) connected to a shock generator (0.6 mA for 1 s) and scrambler (AVS, SCR04; São Paulo). All of the experiments were recorded by a video camera and digitized for further analysis. An ammonium hydroxide solution (0.2%) was used to clean the chamber between trials.
Procedures
The contextual fear conditioning procedure was conducted over 2 days. During the first day, each animal was placed in the observation chamber for an 8 min baseline period. Afterward, three unsignaled 0.6 mA (1 s) electric footshocks were delivered, with an intershock interval of 20 s. The animal was returned to its home cage 2 min after the last footshock. The second day consisted of placing the animal in the same chamber for 8 min where the three footshocks were delivered on the previous day. No footshock or other stimulation occurred during this period. A time-sampling procedure was used to evaluate fear conditioning in response to contextual cues. The animal was observed every 2 s, and a well-trained observer recorded episodes of freezing, defined as the total absence of nonrespiratory movements. The CHL and CLF animals used in the present study were selected from each breeding line based on their freezing response during the test session. The RND animals were chosen randomly.
Two months later, each animal was tested in the same experimental box where contextual fear conditioning occurred and freezing was observed for 5 min. Before testing the animals for contextual fear conditioning, a blood sample was collected to establish baseline plasma corticosterone concentration. The sample was obtained from the lateral tail vein. A total volume of 0.3 ml was collected per rat in heparinized Eppendorf tubes. Twenty minutes after the test, a second blood sample was collected. All of these procedures occurred between 9:00 AM and 1:00 PM. The samples were centrifuged at 1.200 ' g for 15 min at 4°C. The plasma samples were then frozen at -20°C until the assay was performed.
Measurements of plasma corticosterone
Plasma corticosterone concentrations were measured using double-antibody radioimmunoassay in the Laboratory of Neuroendocrinology, School of Medicine, University of São Paulo, Ribeirão Preto, using standard protocols. All of the samples were measured the same assay to avoid interassay variation. The radioimmunoassay for corticosterone required plasma extraction using ethanol. The antibody and standard were provided by Sigma (St. Louis, MO, USA), and the 3H-labeled hormone was obtained from Amersham (Pittsburgh, PA, USA). The lower limit of detection was 2 ng/dl, and the intraassay coefficient of variation was 5%.
Results
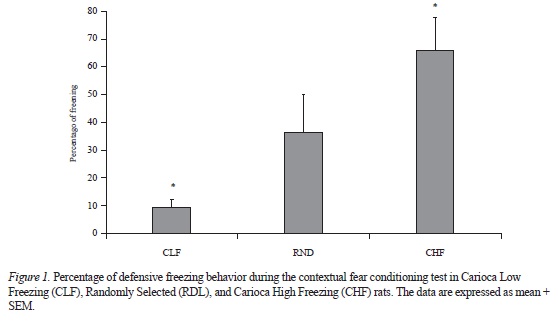
Figure 1 shows the mean (± SEM) percentage of freezing in all groups during the contextual fear conditioning test. A one-way analysis of variance (ANOVA) revealed a significant difference among the three groups (F[218] = 7.16, p <.005). CHF animals exhibited the highest levels of freezing, whereas CLF animals froze the least. The Least Significant Difference post hoc test indicated that CHF and CLF animals were significantly different (p < .005). RND animals exhibited an intermediate level of freezing. The post hoc test showed that RDN and CLF animals were significantly different (p < .05). The difference between RDN and CHF animals was marginal (p = .06).
Figure 2 shows plasma corticosterone concentration before and after contextual fear conditioning. A two-way repeated-measures ANOVA (line' sample time) revealed significant effects of line (F[218] = 10.98, p < .001) and sample time (F[118] = 23.70, p < .001) and a significant line sample time interaction (F [218] = 5.621, p < .05). The post hoc analysis revealed that the basal concentration of corticosterone was higher in CHF rats than in both CLF and RND rats (all p < .05). CHF rats also exhibited higher plasma corticosterone concentrations after the contextual fear conditioning test session compared with CLF and RND animals (all p < .05).
Discussion
Considering that genetics determine much of observed behavior, phenotypic selection is an important tool for understanding the way in which life history interacts with genetic factors. This interaction confers certain vulnerability to stress and anxiety on a given individual. Leon, Landeira-Fernandez, and Cardenas (2009) showed that the same pharmacological treatment (methylenedioxymethamphetamine or fluoxetine) led to opposite effects, depending on whether the subjects were preexposed to chronic mild stress, suggesting that the differential effects of the drug depended on basal conditions. The vulnerability to stress is an important risk factor for developing diseases like anxiety and depression (Aisa et al., 2008; Bisaz, & Sandi, 2012; Castro et al., 2012; Hammen, Marks, Mayol, & deMayo, 1985; Uchida et al., 2008). However, the mere exposure to stressors does not necessarily create vulnerability to psychopathology. In fact, not all individuals respond the same way to the same stressors. Both genetic and environmental factors have been implicated in these susceptibility differences (Castro et al., 2012; Feder, Nestler, & Charney, 2009; Sandi, & Richter-Levin, 2009; Southwick, Vythilingam, & Charney, 2005). Our results support this proposition, suggesting that differences in stress responsivity may be related to phenotypic and genetic (i.e., by continuous phenotypic selection) baseline conditions. For this reason, the same stimulus can trigger different behaviors and hormonal responses.
The present study found that CHF and CLF rats differed in both their basal hormonal profiles and responses to an acute stressful stimulus (i.e., reexposure of the animal to the context in which it was initially conditioned). CHF and CLF rats did not show any differences in their freezing response compared with control rats before the emotional challenge. Moreover, clear differences were observed in their basal concentrations of corticosterone after the emotional challenge. After the emotional challenge, plasma corticosterone levels were greater in CHF rats than in CLF and control rats, suggesting an increase in HPA axis activation and potential vulnerability to stress.
Piazza et al., (1996) found differences in the effect of corticosterone injection in high- and low-responders rats in memory and anxiety tasks. High-responders rats sowed higher locomotricity and increased dopamine release in accumbens when compared to low-responders. However Brink et al. (2008) showed that two different mice strains (C57BL/6J and BALB/c) displayed differences in the formation and extinction of aversive memories. BALB/c had a significant higher release of corticosterone after training and extinction while C57BL/6J did not. They suggested that given that glucocorticoids modulate the processing of aversive stimuli and the reactivity to stress, increase in glucocorticoids is crucial in the formation of aversive memories (Brinks, de Kloet & Oitzl, 2008). This is consistent with the high level of freezing found in CHF two months after the conditioned fear task.
The higher corticosterone concentrations displayed by CHF in both baseline and after stress, correlates to behavioral measures of fear (freezing response). Similar results were reported for Roman High- and Low-avoidance rats strains - phenoty-pically selected by the two way avoidance task. Low-avoidance rats - expressing more anxious behaviors - had significant increases in adrenocorticotropic hormone (ACTH) and corticosterone when compared to High-avoidance rats. However they failed to find baseline differences (Carrasco, Márquez, Nadal, Tobeña, Fernandez-Teruel & Armario, 2008). It should be borne in mind that the rats we used in our experiments are rats that underwent a kind of stressful procedure - contextual fear conditioning. It could be the case that this previous exposure to a stressful event could explain the difference found in basal plasma corticosterone concentration. This suggests that early life events must be taken into account when studying adult behavior. Clinton et al. (2008) showed that exposure to prenatal stress impairs the functioning of the HPA axis.
Our results are also consistent with Lindfors and Lundberg's (2002). They assessed the psychological well-being of young adults and measured plasma cortisol throughout the day. The participants were divided into two groups, those with high psychological well-being scores and those with low scores. Plasma cortisol concentrations were different. The group with high psychological well-being scores had significantly lower cortisol concentrations than the group with low scores (Lindfors, & Lundberg, 2002). In the present study, the basal neuroendocrine profile in CHF and CLF animals may be used as a predictor of the rats' responsivity in the contextual fear conditioning test. Moreover, plasma corticosterone concentrations both under basal conditions and after an emotional challenge were greater in CHF animals than in CLF and control animals. The corticosterone response induced by exposure to the stressor did not significantly change in CLF animals. One possible explanation may be that CLF animals have greater telencephalic regulation of the HPA axis. Further studies are ongoing that seek to determine whether differences exist in the activation of some structures of the prefrontal cortex, such as prelimbic and cingulate regions.
There is growing interest in studying individual differences and "personality" traits in animal models of psychopathology (Castro et al., 2012; Metna-Laurent et al., 2012). Determining baseline traits before any behavioral or pharmacological intervention is becoming crucially important to draw more definitive conclusions. The main conclusion that may be drawn from the present study is that a given intervention, such as the same dose of a pharmacological agent, may lead to completely different results within a sample of subjects. Further studies are needed to determine whether phenotype differences may explain divergent results in behavioral pharmacological research.
Acknowlegments
This work was supported by the "Conselho Nacional de Desenvolvimento Científico e Tecnológico" (CNPq) and the "Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro" (FAPERJ). LLA is recipient of a "Coordenação de Aperfeiçoamento de Pessoal de Nível Superior" (CAPES) doctoral fellowship.
References
Abelson, J. L., Khan, S., Liberzon, I. & Young, E. A. (2007). HPA axis activity in patients with panic disorder: review and synthesis of four studies. Depression and Anxiety, 24, 66-76. [ Links ]
Aisa, B., Tordera, R., Lasheras, B., Del Rio, J. & Ramirez, M. J. (2007). Cognitive impairment associated to HPA axis hyperactivity after maternal separation in rats. Psychoneuroendocrinology, 32, 256-266. [ Links ]
Aisa, B., Tordera, R., Lasheras, B., Del Rio, J. & Ramirez, M. J. (2008). Effects of maternal separation on hypothalamic-pituitary-adrenal responses, cognition and vulnerability to stress in adult female rats. Neuroscience, 154, 1218-1226. [ Links ]
Bardi, M., Rhone, A. P., Franssen, C. L., Hampton, J. E., Shea, E. A., Hyer, M. M., Huber, J. & Lambert, K. G. (2012). Behavioral training and predisposed coping strategies interact to influence resilience in male Long-Evans rats: Implications for depression. Stress, 15, 306-317. [ Links ]
Barlow, D. H., Blanchard, E. B., Vermilyea, J. A., Vermilyea, B. B. & DiNardo, P. A. (1986). Generalized anxiety and generalized anxiety disorder: Description and reconceptualization. American Journal of Psychiatry, 143, 40-44. [ Links ]
Bisaz, R. & Sandi, C. (2012). Vulnerability of conditional NCAM-deficient mice to develop stress-induced behavioral alterations. Stress, 15, 195-206. [ Links ]
Bisaz, R., Schachner, M. & Sandi, C. (2011). Causal evidence for the involvement of the neural cell adhesion molecule, NCAM, in chronic stress-induced cognitive impairments. Hippocampus, 21, 56-71. [ Links ]
Blanchard, D. C. & Blanchard, R. J. (1988). Ethoexperimental approaches to the biology of emotion. Annual Review of Psychology, 39, 43-68. [ Links ]
Blanchard, D. C., Hynd, A. L., Minke, K. A., Minemoto, T. & Blanchard, R. J. (2001). Human defensive behaviors to threat scenarios show parallels to fear- and anxiety-related defense patterns of nonhuman mammals. Neuroscience and Biobehavioral Reviews, 25, 761-770. [ Links ]
Blazevic, S., Colic, L., Culig, L. & Hranilovic, D. (2012). Anxiety-like behavior and cognitive flexibility in adult rats perinatally exposed to increased serotonin concentrations. Behavioural Brain Research, 230, 175-181. [ Links ]
Bolger, N. & Zuckerman, A. (1995). A framework for studying personality in the stress process. Journal of Personality and Social Psychology, 69, 890-902. [ Links ]
Brinks V, de Kloet E. R. & Oitzl, M. S. (2008). Corticosterone facilitates extinction of fear memory in BALB/c mice but strengthens cue related fear in C57BL/6 mice. Experimental Neurology, 216, 375-382. [ Links ]
Carrasco, J., Márquez, C., Nadal, R., Tobeña, A., Fernandez-Teruel, A. & Armario A. (2008). Characterization of central and peripheral components of the hypothalamus-pituitary-adrenal axis in the inbred Roman rat strains. Psychoneuroendocrinology, 33, 437-445. [ Links ]
Castro, J. E., Diessler, S., Varea, E., Márquez, C., Larsen, M. H., Cordero, M. I. & Sandi, C. (2012). Personality traits in rats predict vulnerability and resilience to developing stress-induced depression-like behaviors, HPA axis hyper-reactivity and brain changes in pERK1/2 activity. Psychoneuroendocrinology, 37, 1209-1223. [ Links ]
Feder, A., Nestler, E. J. & Charney, D. S. (2009). Psychobiology and molecular genetics of resilience. Nature Reviews Neuroscience, 10, 446-457. [ Links ]
Fluttert, M., Dalm, S. & Oitzl, M. S. (2000). A refined method for sequential blood sampling by tail incision in rats. Laboratory Animal, 34, 372-378. [ Links ]
Gomes, V. C. & Landeira-Fernandez, J. (2008). Amygdaloid lesions produced similar contextual fear conditioning disruption in the Carioca high- and low-conditioned freezing rats. Brain Research, 1233, 137-145. [ Links ]
Gomez-Lazaro, E., Garmendia, L., Beitia, G., Perez-Tejada, J., Azpiroz, A. & Arregi, A. (2012). Effects of a putative antidepressant with a rapid onset of action in defeated mice with different coping strategies. Progress in Neuropsychopharmacology and Biological Psychiatry, 38, 317-327. [ Links ]
Graeff, F. G. & Zangrossi, H., Jr. (2010). The dual role of serotonin in defense and the mode of action of antidepressants on generalized anxiety and panic disorders. Central Nervous System Agents in Medical Chemistry, 10, 207-217. [ Links ]
Hammen, C. (2005). Stress and depression. Annual Review of Clinical Psychology, 1, 293-319. [ Links ]
Hammen, C., Marks, T., Mayol, A. & deMayo, R. (1985). Depressive self-schemas, life stress, and vulnerability to depression. Journal of Abnormal Psychology, 94, 308-319. [ Links ]
Harris, A. & Seckl, J. (2011). Glucocorticoids, prenatal stress and the programming of disease. Hormones and Behavior, 59, 279-289. [ Links ]
Leon, L. A., Landeira-Fernandez, J. & Cardenas, F. P. (2009). Effects of chronic intracerebroventricular 3,4-methylenedioxy-N-methamphetamine (MDMA) or fluoxetine on the active avoidance test in rats with or without exposure to mild chronic stress. Behavioural Brain Research, 205, 259-264. [ Links ]
Lindfors, P. & Lundberg, U. (2002). Is low cortisol release an indicator of positive health? Stress and Health, 18, 153-160. [ Links ]
McNaughton, N. & Corr, P. J. (2004). A two-dimensional neuropsychology of defense: Fear/anxiety and defensive distance. Neuroscience and Biobehavioral Reviews, 28, 285-305. [ Links ]
Metna-Laurent, M., Soria-Gomez, E., Verrier, D., Conforzi, M., Jego, P., Lafenetre, P. & Marsicano, G. (2012). Bimodal control of fear-coping strategies by CB1 cannabinoid receptors. Journal of Neuroscience, 32, 7109-7118. [ Links ]
Oitzl, M. S., Champagne, D. L., van der Veen, R. & de Kloet, E. R. (2010). Brain development under stress: hypotheses of glucocorticoid actions revisited. Neuroscience and Biobehavioral Reviews, 34, 853-866. [ Links ]
Piazza, P. V., Rougé-Pont, F. V., Deroche, V., Maccari, S., Simon, H. & Le Moal, M. (1996). Glucocorticoids have state-dependent stimulant effects on the mesencephalic dopaminergic transmission. Proceedings of the National Academy of Sciences of the United States of America, 93, 8716-8720. [ Links ]
Raber, J., Akana, S. F., Bhatnagar, S., Dallman, M. F., Wong, D. & Mucke, L. (2000). Hypothalamicpituitary-adrenal dysfunction in Apoe-/- mice: Possible role in behavioral and metabolic alterations. Journal of Neuroscience, 20, 2064-2071. [ Links ]
Reis, F. M., Albrechet-Souza, L., Franci, C. R. & Brandão, M. L. (2012). Risk assessment behaviors associated with corticosterone trigger the defense reaction to social isolation in rats: Role of the anterior cingulate cortex. Stress, 15, 318-328. [ Links ]
Sandi, C. (2004). Stress, cognitive impairment and cell adhesion molecules. Nature Reviews Neuroscience, 5, 917-930. [ Links ]
Sandi, C., Cambronero, J. C., Borrell, J. & Guaza, C. (1992). Effects of HPA hormones on adapted lymphocyte responsiveness to repeated stress. Brain Research Bulletin, 28, 581-585. [ Links ]
Sandi, C. & Richter-Levin, G. (2009). From high anxiety trait to depression: A neurocognitive hypothesis. Trends in Neurosciences, 32, 312-320. [ Links ]
Southwick, S. M., Vythilingam, M. & Charney, D. S. (2005). The psychobiology of depression and resilience to stress: Implications for prevention and treatment. Annual Review of Clinical Psychology, 1, 255-291. [ Links ]
Uchida, S., Nishida, A., Hara, K., Kamemoto, T., Suetsugi, M., Fujimoto, M., Watanuki, T., Wakabayashi, Y., Otsuki, K., McEwen, B. S. & Watanabe, Y. (2008). Characterization of the vulnerability to repeated stress in Fischer 344 rats: Possible involvement of microRNA-mediated down-regulation of the glucocorticoid receptor. European Journal of Neuroscience, 27, 2250-2261. [ Links ]