Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Earth Sciences Research Journal
Print version ISSN 1794-6190
Earth Sci. Res. J. vol.15 no.2 Bogotá Jul./Dec. 2011
Zeolitisation of Neogene sedimentary and pyroclastic rocks exposed in Paipa (Boyacá), in the Colombian Andes: simulating their natural formation conditions
Fredy Alberto Quintero Ortíz, Johel Torres Valenzuela and Carlos Alberto Ríos Reyes
Escuela de Geología, Universidad Industrial de Santander, Bucaramanga, Colombia. E-mail: carios@uis.edu.co
Record
Manuscript received: 10/04/2011 Accepted for publications: 25/11/2011
ABSTRACT
The present study concerns the synthesis of zeolites from Neogene sedimentary and pyroclastic rocks exposed around the Sochagota Lake, Paipa (Boyacá). Two synthesis methods were used: conventional hydrothermal treatment and alkaline fusion followed by hydrothermal reaction. Both raw materials and synthesised zeolytic products were characterised by X-ray diffraction (XRD), Fourier transform infrared spectroscopy (FTIR) and nuclear magnetic resonance (NMR). Several zeolytic phases were synthesised, including faujasite (FAU), phillip-site (PHI) and sodalite (SOD). The results showed that the alkaline fusion approach was more efficient regarding hydrothermal conversion of the raw materials than conventional hydrothermal treatment, taking into account that zeolytic products having a higher degree of crystallinity and few impurities were obtained in this way. This study was aimed at applying experimental mineralogy to a laboratory simulation of the geological conditions in which zeolites can occur as the basis for defining criteria for exploring natural zeolites in Colombia, with prospects for the profitable exploitation of these mineral resources in different parts of Colombia.
Keywords: Sochagota Lake, Paipa, synthesis, hydrothermal reaction, zeolites, experimental mineralogy, natural formation conditions.
RESUMEN
El presente estudio se refiere a la síntesis de zeolitas a partir de rocas sedimentarias y piroclásticas del Neógeno que afloran alrededor del lago Sochagota, Paipa (Boyacá). Dos métodos diferentes de síntesis se utilizaron: (1) tratamiento hidrotérmico convencional y (2) fusión alcalina seguida por reacción hidrotérmica. Tanto las materias primas, como los productos zeolíticos sintetizados se caracterizaron por difracción de rayos X (DRX), espectroscopia infrarroja con transformada de Fourier (IRTF) y resonancia magnética nuclear (RMN). Diferentes fases zeolíticas fueron sintetizadas, las cuales incluyen faujasita (FAU), filipsita (PHI) y sodalita (SOD). Los resultados muestran que la incorporación de la fusión alcalina fue más eficiente en la conversión hidrotérmica de las materias primas que el tratamiento hidrotérmico convencional, teniendo en cuenta que los productos zeolíticos mostraron mayor grado de cristalinidad y baja cantidad de impurezas. La contribución de este estudio fue aplicar la mineralogía experimental para simular a escala de laboratorio las condiciones geológicas y composicionales que pueden relacionarse con la formación de zeolitas en un contexto geológico específico, aspectos relevantes para definir criterios de exploración de zeolitas naturales en Colombia con perspectivas de explotación rentable de estos recursos minerales en diferentes regiones del país.
Palabras claves: Lago Sochagota, Paipa, síntesis, reacción hidrotérmica, zeolitas, mineralogía experimental, condiciones naturales de formación.
Introduction
Zeolites are crystalline aluminosilicates having open 3D framework structures consisting of SiO4 and AlO4 tetrahedra linked to each other by oxygen atoms to form regular intracrystalline cages and channels having molecular dimensions. The lattice's negative charge is neutralised by a positive charge from cations located within the material's pores; they also contain water and/or other molecules within their pores. Zeolites were first described from vugs and fissures in basaltic flows in the mid-eighteenth century and they are now known to be widespread in a variety of geological environments.
Zeolites may occur naturally as authigenic minerals formed from volcanic glass and various rock-forming minerals by interaction with aqueous solution or fluid in many geochemical environments (Breck, 1974; Mumpton, 1977; Gottardi and Galli, 1985; Hay, 1986; Boles, 1988). More zeolite species occur in vesicles and fissures in basic volcanic rocks than in any other geologic setting. However, zeolites from sedimentary rocks in particular, represent the most important occurrences, both in terms of areal extent of deposits and abundance of certain zeolite species (Langella et al., 2001). Others may be obtained from laboratory synthesis for specific commercial uses and for improving the understanding of their geological occurrence. Zeolites have unique porous properties and are used in various applications in petrochemical cracking, ion-exchange (water softening and purification), separation and the removal of gases and solvents; other applications would include agriculture, animal husbandry and construction.
Many experimental studies concerning zeolite synthesis have been conducted to clarify the geological conditions regarding zeolite formation, using different starting materials in varied chemical and temperature conditions (Breck, 1974; Gottardi and Galli, 1985). Zeolites can be readily synthesised in hydrothermal conditions and a lot of information is readily available concerning synthetic zeolites and chemical resolution for zeolite synthesis (Hawkins, 1981). The chemical preparation of synthetic zeolites from silica and alumina is expensive; zeolite researchers are thus seeking cheaper raw materials for zeolite synthesis to reduce costs, including clay minerals such as kaolinite (Breck, 1974; Barrer et al., 1974; Boukadir et al., 2002), halloysite (Klimkiewicz and Drag, 2004), illite, smectite, interstratified illite-smectite (Baccouche et al., 1998), montmorillonite
(Cañizares et al., 2000) and bentonite (Boukadir et al., 2002; Ruiz et al., 1997), natural zeolites (Kang and Kazuhiko, 1997; Watanabe et al., 2005; Covarrubias et al., 2006), volcanic glasses (Breck, 1974; Barrer, 1982; Vitarelli et al., 1983; Colella et al., 1985; Moirou et al., 2000), diatomite (Anderson et al., 2005; Holmes et al., 2001), high silica bauxite (Puerto and Benito, 1996), oil shale (Shawabkeh, 2004) and natural clinker (Ríos and Williams, 2008; Ríos et al., 2008; Sandoval et al., 2009).
The present study reports the laboratory simulation of the hydro-thermal alteration of Neogene sedimentary and pyroclastic rocks exposed around the Sochagota Lake (Paipa, Boyacá) to zeolites to understand the geological conditions controlling their formation. Colombia has many volcanic areas; however, unlike other regions worldwide, there are no reports about the occurrence of natural zeolites, except in Cretaceous volcanic rocks from the Timba basaltic flows and sills and Barroso-Amaime complex (Nivia, 1989; Nivia et al., 2006). The geological similarity between several Colombian regions, such as Paipa, and those reported in other parts of the world (Taylor & Surdam, 1981; Ghiara et al., 1999; Manassero et al., 2000; Weisenberger & Spürgin, 2009) would suggest that Colombia has zeolite deposits; such similarities concern tectonic setting, type of volcanism and deposits and the occurrence of explosive rhyolitic and trachytic volcanic rocks having alkaline to calcoalkaline affinity.
Zeolites as products of hydrothermal crystallisation are generally known from active geothermal systems associated with volcanic rocks. Pardo et al., (2005, and references therein) have reported the occurrence of a geothermal system in Paipa. However, zeolite deposits of economic interest have not been discovered in Colombia and no related research has been carried out until now. This study identifies such lack as being the main gap in knowledge which may hinder our ability to fully understand how natural zeolites form. Likewise, few studies deal with zeolite synthesis from geomaterials. The main purpose of the present work is to contribute towards understanding zeolitisation of sedimentary and pyroclastic rocks in Paipa (Boyacá) in the Colombian Andes and the formation of zeolite deposits in Colombia.
Laboratory simulations were thus used to transform them in hydrothermal conditions to better understand and explain the possibilities of conversion in natural geological environments. One of the most exciting developments of this work focused on the hydrothermal zeolitisation of sedimentary and pyroclastic rocks. Developing future work on zeolite synthesis could be very important for Colombia and zeolite researchers in designing materials in many areas of technology for specific environmental applications, particularly referring to using zeolites in removing contaminants and toxic components from the environment.
Experimental procedure
Materials and chemical reagents
Neogene sedimentary and pyroclastic rocks outcropping around the Sochagota Lake in Paipa formed the starting materials for our experiments (Figure 1). The Tilatá formation (NgQt) out crops in the lowlands near the Sochagota Lake; it consists of fine- to coarse-grained quartz sandstones, interbedded with siltstones, claystones and sandy conglomerates. Pardo et al., (2005) have identified trachyandesite to alkaline rhyolitic ignimbrites, pyroclastic surge, block and ash flow deposits and volcaniclastic deposits, mapped as NgQv and related to Plio-Pleistocene, highly explosive volcanism. Several aspects regarding the occurrence of these rocks are illustrated in Figure 2. The geological raw materials were prepared prior to synthesis, using the following steps: rough crushing with a Retsch Jaw Crusher BB 200 to - 2mm, milling with a Retsch RM100 mortar grinder mill to clay particle size and sieving with a 200 mesh Ro-Tap sieve shaker; 63 µm particles were selected for zeolite synthesis. 99% analytical grade sodium hydroxide pellets (Aldrich Chemical Company, Inc.) and distilled water were used in all experiments.
Zeolite synthesis
The synthesis of zeolytic materials was investigated by using conventional hydrothermal synthesis and alkaline fusion prior to hydrothermal synthesis, taking into account that direct treatment of the raw materials using alkaline solutions could be quite time-consuming, energy-intensive and/or environmentally-unfriendly, and could be overcome by using the alkaline fusion approach. During classic hydrothermal synthesis, calculated amounts (0.96 g and 2.97 g) of NaOH pellets were added to distilled water in plastic reaction beakers (250 ml) to prepare NaOH solutions at different concentrations (1 and 3 M). The raw materials were then added to the alkaline solutions in an 8 ml/g alkaline solution/raw material ratio. An alkaline fusion step was incorporated in the second method prior to the hydrothermal treatment to enhance the hydrothermal conditions for zeolite synthesis, considering the large amounts of aluminosilicates which can be dissolved by using this method.
First, 6.20 g of raw material and 7.44 g of NaOH (pellets) in raw material/alkaline activator (1/1.2 weight ratio) were dry mixed in a mortar grinder mill for 30 min and the resultant mixture was fused at 600°C for 1 h. The fused product was ground in an agate mortar and then 4.40 g of the product was dissolved in 21.50 ml distilled water (1/4.9 ratio) in plastic reaction beakers (250 ml) with constant stirring to form the amorphous precursors. The progressive addition of reagents in both methods involved stirring them until their dissolution homogenised the reaction gels.
The amount of reagents used for preparing the hydrogels was based on previous experimental work developed by Ríos et al., (Ríos et al., 2007; Ríos and Williams, 2008, 2010; Sandoval et al., 2009; Ríos et al., 2008, 2009a, 2009b, 2010a, 2010b, 2011). Hydrogels were aged in static conditions for 24 h. The hydrothermal reaction was carried out in static conditions, transferring the hydrogels to 65 ml teflon (polytetrafluoroethylene - PTFE) bottles (Cowie Technology Ltd) for preparations heated at the required temperature (80°C and 100°C) for different reaction times (24, 48, 96 h). The reactors were removed from the oven at the scheduled times and quenched in cold water to stop the reaction; hydrogel pH was measured before and after hydrothermal treatment. The reaction mixtures were then filtered and washed with distilled water to remove excess alkali. The samples were oven-dried at 80°C overnight and then the dried samples were weighed and kept in plastic bags for characterisation.
Characterising the raw materials and synthesised zeolites
Powder X-ray diffraction patterns for the raw materials and synthesised products were recorded on a Philips PW1710 diffractometer operating in Bragg-Brentano geometry with Cu-Ka radiation (40 kV and 40 mA) and secondary monochromation. Data was collected in the 3-50° 29 range (0.02° step size). The crystalline patterns were compared with the standard line patterns from the Powder Diffraction File database supplied by the International Centre for Diffraction Data (ICDD), with the help ofJoint Committee on Powder Diffraction Standards (JCPDS) files for inorganic compounds. The solid phases' morphological structure was examined by scanning electron microscopy (ZEISS EVO50) and the mineral phases' chemical composition was studied using the EDXS mode in the following analytical conditions: I probe 1 nA, EHT=20.00 kV, 100 uA beam current, Signal A=SE1, WD=8.0 mm. Fourier transform infrared (FT-IR) spectra were collected between 4,000 and 400 cm-1 on a Bruker Tensor 27 FT-IR spectrometer in KBr media for investigating zeolite framework vibrations. A couple of milligrams of sample were mixed with about 200 mg of KBr powder. The mixture was pressed into a tablet which wass then used in the analysis.
Results and Discussion
Starting materials
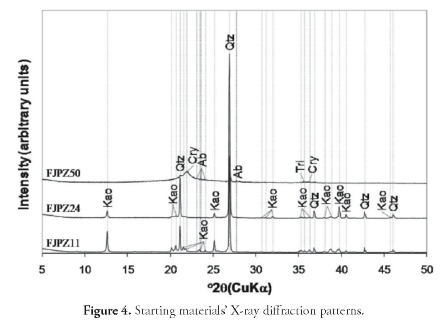
A description of the macroscopic characteristics of the raw geomaterials selected for zeolite synthesis (Figure 3) is now presented. Briefly, the FJPZ11 sample had a weathered white, fissile and compact aspect with no stratification. It was classified as very fine- to fine-grained sandstone in which 5%-25% of the sand grains were composed of feldspar, being white, muddy, matrix-supported as a result of kaolin forming after feldspar. The FJPZ24 sample was also very fine- to fine-grained sandstone having a high feldspar content, being white, muddy, matrix-supported (due to kaolinitisation of feldspar). The FJPZ50 sample was classified as a volcanic tuff, a pyroclastic geomaterial formed as fragmental products of volcanic eruptions, having feldspar phenocrystals, in a white, vesicular, volcanic glass matrix. It consisted of very poorly-sorted pumice fragments and very angular lithics (2-64 mm range), chaotically distributed in a white, vesicular, volcanic glass and porous matrix. The X-ray powder diffraction patterns for the starting materials are shown in Figure 4, revealing high-intensity reflections attributed to quartz and kaolinite, although they also showed the presence of albite, crystoballite and tridymite. The tuff was characterised by the occurrence of an amorphous aluminosilicate (see the broad hump at 29 = 17.5-20.5°, having a maximum at 29 = ~22o). It also had a strong 26.6° diffraction peak at 29, which was attributed to quartz.
Synthesised zeolites
X-ray diffraction
Zeotypes were identified by comparing the diffraction pattern to the International Centre for Diffraction Data's powder diffraction file (PDF) database. Our data indicated the formation of-phillipsite (PDF 000-511497), sodalite (PDF 010-88-1190) and faujasite (PDF 000-39-1380).
Hydrothermal transformation of geomaterials in 1 M NaOH solutions. The XRD patterns in Figure 5 show the progressive synthesis of zeolite by the conventional hydrothermal method, using solutions of 1 M NaOH. This revealed a very small reduction of the starting materials' characteristic peaks and the appearance of weak reflection peak intensity corresponding to traces of phillipsite-type zeolite, indicating low activation efficiency. The FJPZ11 sample had constant peaks corresponding to quartz and kaolinite, but phillipsite appeared (after 24 hours) with increasing reaction time. The only phase formed for the FJPZ24 sample was phillipsite after 96 hours.
The activation of the FJPZ50 sample was unsuccessful, as no zeolite phase became formed, and only a decrease in crystoballite peak intensity and increase in characteristic quartz peaks were observed, although the latter decreased after 96 hours.
Hydrothermal transformation of geomaterials in 3 M NaOH solutions. The XRD pattern in Figure 6 shows that increasing NaOH solution concentration favoured the formation of sodalite- and phillipsite-type zeolites, with a progressive decrease in peak intensity for the phases related to the minerals present in the starting materials. The products resulting from the synthesis always had starting materials' relict phases. A progressive kaolinite dissolution was observed for the FJPZ11 sample between 6 and 24 hours of reaction, disappearing after 96 hours. The characteristic quartz peak had decreased intensity, indicating this mineral's stability during hydrothermal treatment. Moreover, the progressive dissolution of starting material's mineral phases was accompanied by the appearance of sodalite after 6 hours. A progressive increase in the intensity of peaks characteristic of this zeolytic phase was observed between 6 and 96 hours. Activation of the FJPZ24 sample, produced similar results to those observed in the previous case, except for the occurrence of sodalite having weak reflection peaks. Using the FJPZ50 sample as starting material, a progressive loss of its amorphous character turned it into a synthesis product characterised by the appearance of relative poorly crystalline phillipsite only after 96 hours. In this case, an increase in characteristic quartz peak intensity and the presence of other relictic phases such as albite was observed in the products so obtained.
Transformation of geomaterials by alkaline fusion followed by hydrothermal treatment. Zeolite synthesis by this method revealed that incorporating an alkaline fusion step promoted the dry reaction between the mineral phases present in the raw geomaterials and the alkaline activator. As shown in Figure 7, there was an almost complete transformation of the starting materials into high purity faujasite-type zeolite and phillipsite, although relictic phases (quartz and albite) of the starting materials still remained in the synthesis products. The alkaline-fused products were sodium silicate and aluminate salts. Newly-formed compounds dissolved in water more readily than the mineral phases in the starting materials, which had low dissolution speed, some of them occurring as relict phases in the synthesis products.
The activation of the FJPZ11 sample produced a rapid dissolution of the alkaline-fused products only after 6 hours reaction, accompanied by a gradual decrease in the intensity of characteristic quartz peaks which persisted in the products so obtained. Moreover, the occurrence of faujasite was recorded after 24 hours, showing an increase in the intensity of characteristic peaks between 24 and 96 hours. The activation of the FJPZ24 sample produced similar results to those observed in the previous case, except for the fact that faujasite had less intense peaks. The transformation of the tuff (FJPZ50 sample) was characterised by the loss of the starting material's amorphous nature, with progressive dissolution of the alkaline-fused products, except for the gradual increase of characteristic quartz peak intensity, with the occurrence of phillipsite along with quartz and albite in the synthesis products. Phillipsite was formed after 24 hours, showing an increase in its characteristic peak intensity with the reaction time.
Scanning electron microscopy
SEM images of the morphology of the synthesised zeolytic products obtained after activating the starting materials are shown in Figure 8, revealing a change in the morphology of the original surface of raw geomaterial particles (blocky morphology, not shown here) to the modified product's far rougher surface. The zeolytic products obtained were characterised by the occurrence of phillipsite, sodalite and faujasite. Figure 8a illustrates complex aggregates of large clusters of radiating tetragonal prisms of phillipsite. Sodalite occurs as bladed crystals having a "cotton-ball"-like morphology (Figure 8b). The typical morphology of well-developed octahedral faujasite crystals displaying interpenetration-twinning is shown in Figure 8c.
Fourier transform infrared spectroscopy
Based on the results of the X-ray diffraction analysis, the FJPZ24 sample was selected to confirm its transformation during synthesis in hydrothermal conditions and the presence of an aluminosilicate framework in the zeolites, which was shown in the FTIR spectra (Figure 9). The starting material's characteristic peaks became less pronounced in some regions, resulting in their disappearance, although sometimes they only became weakened in intensity. Characteristic mineral phase vibrations present in both the starting material and synthesis products were observed in the present study; however, in other cases, some vibration bands moved toward higher or lower frequencies. The bands at 3,659.22, 3,620.68 and 3,456.36 cm-1 corresponded to oxygen-hydrogen bond vibration frequencies belonging to the water molecules retained in the starting material. Kaolinite's characteristic vibrations were observed at 1,115.34, 1,029.56 (surface O-H bond bending), 917.14 (internal bending O-H bond), 669.49 and 540.04 cm-1. The mid infrared silicate spectra in the 1,200400 cm-1 range were classified into four characteristic bands of around 1,000, 780, 695 and 450 cm-1 regarding the standard quartz spectra (Saikia et al., 2008). According to Saikia et al., (2008), among these four characteristic peak regions, a peak at 695 cm-1 is unique to crystalline materials, which however was not observed in the sample analysed here. The 1,029.56 (asymmetric T-O stretching vibrations) and 788.68 (symmetric T-O stretching vibrations) cm-1 bands were characteristic for quartz. New vibration bands appeared concurrently with the gradual disappearance of the starting material's characteristic bands, which may have been attributed to zeolytic materials. Zeolites frameworks vibrations give rise to typical bands in mid and far infrared spectra. A distinction was made between TO4/2 tetrahedra external and internal vibrations (with, for example, T = Si or Al). The original assignments for the main IR bands (Flaningen et al., 1971) were as follows: the 1,250-950 cm-1 region was attributed to the tetrahedral asymmetric stretching zone's inner bonds and the asymmetric stretching of external bonds between tetrahedral zones. The band at 995.79 cm-1 corresponded to asymmetric stretching in faujasite. Sodalite occurrence was confirmed by the bands at 991.08 and 1,012.31 cm-1
There was no evidence of IR absorption in the 820-750 cm-1 range assigned by Flaningen et al., (1971) to the stretching vibrations of external bonds between tetrahedra. There was a poorly defined band at 682.51 cm-1 in the 750-650 cm-1 spectral zone which could be attributed to symmetric stretching in faujasite. There were two well-defined bands at 709.81 and 690 cm-1, which were in good agreement with the bands for sodalite reported by Flaningen et al., (1971). The bands in the 650-500 cm-1 region were related to the presence of double rings (D4R and D6R) in the zeolytic materials' framework; the band at 557.42 cm-1 corresponded to the hexagonal double ring vibration which is attributed to faujasite. There was no evidence of bands corresponding to sodalite, which is characteristic for this zeotype, taking into account that it does not have a double-ring framework like faujasite. There was no evidence of IR absorption in the 500-450 cm-1 region related to O-T-O bond deformation vibrations (Flaningen et al., 1971), except for a vibration band around 450 cm1. There was no evidence of the well-defined symmetric bands reported for sodalite in previous studies (Flaningen et al., 1971). Table 1 shows the IR spectral data for the synthesised zeolites.
29Si and 27Al nuclear magnetic resonance
Figure 10 shows the NMR spectra for starting material 29Si and 27Al (FJPZ24 sample) and synthesised products. The starting material's 29Si spectrum (Figure 10a) had two well-resolved signals at -91.13 and -107.23 ppm attributed to the existence of two different silicon sites and revealed the presence of kaolinite and quartz, respectively, as reported in previous studies (Barron et al., 1983; Thompson and Barron, 1987; Hayashi et al., 1992; Letaief et al., 2006). Activated starting material's chemical shift values for 29Si spectra are illustrated in Figures 10b-10d, indicating the occurrence of different silicon sites for the zeolytic phases so obtained, and relics of kaolinite and quartz. The 27Al spectrum (Figure 10a) consisted of a simple resonance at 1.23 ppm, which was assigned to octahedrally coordinated Al in kaolinite and quartz. The 27Al spectra for the treated geomaterial (Figures 10b-10d) had resonance signals at 1.23 ppm, attributed to octahedral Al (corresponding to relictic phases), and at 57.86 and 60.79 ppm, attributed to tetrahedral Al.
The experimental method used in zeolite synthesis played an important role in determining the zeotype to be formed. An alkaline fusion step was carried out prior to the hydrothermal treatment because it plays an important role in enhancing the hydrothermal conditions for zeolite synthesis. According to Ríos & Williams (2008), synthesis chemistry becomes subject to perturbations caused by the presence of impurities in the starting materials, which may remain insoluble during crystallisation and make undesired species nucleate, thereby developing mixtures of different zeolytic materials coexisting in the synthesis products, and the dominant crystalline phase depending on formation conditions.
Simulating geological conditions for zeolite formation
Experimental mineralogy was used for simulating zeolite formation conditions in a specific geological context such as Paipa, where explosive rhyolitic and trachytic volcanic rocks having alkaline and calcoalkaline affinity occur, to ascertain in which conditions and from what compounds their formation may occur. Most of the exploitable natural zeolite deposits in the world are found in initially glass-rich, clastic formations of volcanic origin, frequently being associated with acid volcanism. According to Gottardi (1989), these deposits form as a result of the slow percolation of meteoric water through volcanic tuff beds, becoming more alkaline and saline with depth, thus raising its pH. This leads to zonal variation of water chemistry reacting with the host rock, which is expressed in the mineral product's vertical zonation. Thus, the volcanic glass is altered to clay minerals at the top and different types of zeolites form below. Authigenic alkali feldspars crystallise at greater depth. The same vertical zonation could be established in the laboratory by transforming the proposed starting materials and simulating hydrothermal conditions similar to those found in this geological environment.
Zeolites can be readily synthesised in a few hours in hydrothermal conditions and there is a lot of information regarding zeolite synthesis solution chemistry (Hawkins, 1981). However, a major problem concerns zeolite synthesis at high temperatures and pressures regarding the formation of zeolites in nature (Sand, 1980). According to Hawkins (1981), this can be addressed if zeolites typical of low-temperature conditions are synthesised from natural reactants in chemical conditions similar to those in which natural minerals are thought to be formed. The synthesis of zeolytic materials from zeolite-absent rocks demonstrates that such simulation may reproduce the overall geological conditions in which these mineral phases may form in nature. The transformation of aluminosilicate geomaterials in zeolytic phases can be explained by two main stages: the dissolution of raw geomaterials' aluminosilicate phases or their alkaline-fused product releasing Si and Al and zeolite crystallisation.
An attempt was first made to destroy the structure of the aluminosilicates present in the starting materials by separating the silicon tetrahedra and aluminium octahedra which finally re-crystallised in several zeolite frameworks. Geomaterials' transformation in zeolites reflects mineralogical, textural and chemical responses to the starting materials in hydro-thermal conditions. Undoubtedly, the mineralogical composition of the aluminosilicate-rich starting material played an important role, as well as the presence of elements other than Si and Al, which promoted the production of different types of zeolites and undesirable mineral phases. Very low Fe and Ca content can control synthesis, taking into account that Febearing minerals can have inert behaviour and Cabearing phases can act as a zeolite synthesis inhibitor (Juan et al., 2007).
The glass phase is also very important during zeolitisation because it easily dissolves into the alkaline solution compared to crystalline phases like quartz or feldspar, which generally persist in synthesis products. Although the initial mineralogical composition influences hydrothermal reaction product mineralogy, its effect will be minor compared to the effect produced by factors such as solution permeability, temperature and composition (pH). However, the major input of chemical constituents into aqueous systems comes from mineral dissolution, and, therefore, knowledge of mineral solubility and their mode and rate of dissolution is essential for interpreting water-rock interaction and the transport of dissolved solids in water systems (Stefánsson, 2001). Zeolite formation by hydrothermal weathering varies according to the nature and the origin of the weathered rocks. In nature, the origin of zeolites is related to the hydrothermal transformation (hydrolysis) of glass to smectite, prior to zeolite formation, which was not reported in the study area probably related to the requirements for large-scale zeolitisation, such as a high percentage of glassy particles, high permeability and favourable hydrological conditions (Manassero et al., 2000).
On the other hand, during rock-solution interaction, differences in the reaction products, crystallisation sequences, and reaction kinetics seem to be related to several intensive variables such as interaction time, hydrological system (closed or open), temperature, pH, contact solution chemical composition and starting materials (e.g. Barth-Wirsching and Holler, 1989). The reasons are diverse and related to aspects such as volcanic processes, as well as other less understood ones, including the relationship between the zeolitisation of pyroclastic rocks and the formation of natural zeolites. The intention was to produce synthetic zeolites in a short time and at the lowest possible temperature, which is very important for geochemists whose aim is to reproduce synthetic zeolite formation in the laboratory in hydrothermal conditions and obtain better understanding of natural zeolite formation in a specific geological context.
The alteration of Neogene sedimentary and pyroclastic rocks exposed in the Paipa area was thus evaluated by alkaline activation at low temperatures and autogenous pressure using two different methods, which are described below. This approach was also interesting because it involved hydrothermal alteration of primary minerals or rocks into zeolites, although transformation was generally slow and the synthetic zeolites crystallised with other phases. Therefore, zeolite synthesis in the laboratory requires the verification of the geological conditions involved in the formation of this group of minerals. Colombia has a number of volcanic areas within which natural zeolites may be found. However, it was evident from this study that it is not enough to just determine geological similarity and pyroclastic volcanic regions between Colombia and other parts of the world where zeolites have been reported but also to determine areas where volcanic rocks have reacted with alkaline groundwater. There is thus a connection between the type of lithology and type of site where natural zeolites should be sought.
Exploration strategies
Different descriptive and genetic geological models have been applied to the exploration of mineral deposits which are based on the geological setting, host rock lithology, form of the deposit, economic minerals, the mineralogy of ore and host rocks and geochemical and geophysical characteristics common to a number of similar mineral deposits presumed to have been formed by the same genetic process. This study was intended to define exploration criteria for natural zeolites in volcanic terrain like Paipa (Colombia).
Recent studies (e.g., Taboada et al., 2000) have proposed that volcanic rocks occuring in Paipa originated from the Caribbean plate's subduction. Pardo et al., (2005) have identified trachyandesitic to alkaline rhyolitic ignimbrites, pyroclastic surge, block and ash flow deposits and volcaniclastic deposits in this area which are related to Plio-Pleistocene, highly explosive volcanism. However, it is also common that extensive lacustrine deposits represented by intercalated tuffs, siltstones, diatomites and sandstones occur in such geological setting (Brathwaite, 2003, 2006). Diatomite may occur down or up stratigraphically from lacustrine tuffs (Brathwaite, 2006) and has been reported in several parts of Colombia, although there is no report of its appearance in the study area. The main zeolite occurrences are in tuffs and volcaniclastic rocks in lakes or deep sea basins (Hay and Sheppard, 2001), although they are also common in low temperature hydrothermal alteration zones in geothermal and epithermal systems (Utada, 2001). According to Alfaro et al., (2010) the Paipa volcano has an active hydrothermal system as inferred from surface manifestations and an inactive volcano-magmatic system. The Paipa-Iza Fault is interpreted as a basement structure related to a previous extensional tectonic phase which became reactivated during Andean uplift, preserving the nature of open breaks facilitating hydrothermal fluid circulation.
The zeolite deposits are located on or close to faults, indicating that the faults acted as feeders for heated alkalichloride water that caused the deposition of the zeolite minerals (Brathwaite, 2006). There is thus a spatial association with geothermal systems and faults in the volcanic area of Paipa to provide a source and conduit for heated alkaline solutions which react with vitric tuffs to form the zeolites. Hydrothermal eruption breccias, sinter overlying zeolytic tuffs and hydrothermal adularia in permeable fault zones are indicators of hydrothermal activity that may have formed zeolites, as suggested by Brathwaite (2006).
No evidence has been presented in this study to support the occurrence of all these requirements, meaning that they cannot be used as exploration guides. According to Brathwaite (2006), geophysical signatures have provided useful information on zeolite deposits which can be associated with broad magnetic lows within which there are coincident gravity and resistivity lows. The mineralogy of hydrothermal alteration is one of the most useful geological exploration criteria for natural zeolites. A start can be made in what would be a geological curiosity using different analytical techniques, such as X-ray diffraction and scanning electron microscopy. However, using microthermometry of fluid inclusions potentially provides key information on the genesis of most ore deposits and cannot be replaced by other techniques as a guide for mineral exploration (Cam-prubí, 2010). The following exploration criteria for zeolite deposits in a geological setting such as Paipa are thus suggested:
• lacustrine vitric tuff host rocks provide large volumes of reactive volcanic glass;
• diatomite occurring down or up stratigraphically from lacustrine tuffs;
• a spatial association with geothermal systems and faults to provide a source and conduit for heated alkaline solutions;
• geophysical signatures having coincident gravity and resistivity lows within broad magnetic lows representing hydrothermal alteration zones;
• mineralogy of the hydrothermal alteration; and
• mineralising fluids.
Geology undoubtedly plays a very important role in natural zeolite exploration. However, different zeotypes and their favourable geologic settings and controls must be understood and detected on a regional or local scale. On the other hand, the application of several analytical techniques would provide valuable information to be integrated with geology. According to Robert et al., (2007), a successful strategy should emphasise detecting geological features typical of favourable settings and hydrothermal manifestations of deposits, such as alteration and mineralisation.
Conclusions
Zeotypes like phillipsite, sodalite and faujasite zeolitisation having very few impurities (quartz and feldspars) were successfully synthesised from Neogene sedimentary and pyroclastic rocks outcropping in Paipa (Boyacá) in the Colombian Andes. The experimental method used in the synthesis played an important role in determining the zeotype to be formed. The alkaline fusion approach was more effective than the conventional hydrothermal treatment in transforming the starting materials. Zeolite synthesis chemistry was subject to perturbations caused by the presence of impurities in the starting material, which remained insoluble during crystallisation and made undesired species nucleate, thereby developing mixtures of different zeolytic materials coexisting in the synthesis products.
Further studies should be carried out in well-optimised experimental conditions using different alkaline solutions to compare the effect of the activating agent on starting materials' transformation and to better understand mineral alteration in hydrothermal conditions. Simulation conditions to be used in zeolite synthesis should thus reproduce the origin of natural zeolites as a process related to the hydrothermal transformation of glass to smectite, prior to the formation of zeolites, which could not be proved in this study. It is therefore very important to consider differences in reaction products in the rock-solution interaction, as well as crystallisation sequence and reaction kinetics, which seem to be related to several intensive variables. This research provides valuable information for defining geological exploration criteria in geological settings similar to those in which zeolites have been reported in other parts of the world to focus studies concerning the exploration of natural zeolites in Colombia.
Acknowledgments
The authors gratefully acknowledge the Universidad Industrial de Santander for financially supporting the fieldwork. This study has benefited from research facilities provided by the following institutions: Universidad Industrial de Santander, University of Wolverhampton, University of Durham and Instituto Zuliano de Investigaciones Tecnológicas. We would like to thank Mr. Miguel Ramos, Mrs. Barbara Hodson, Mr. Alejandro Torres and Dr. David Apperley for assistance with XRD, SEM, FTIR and NMR data acquisition, respectively.
References
Alfaro, C., Velandia, F., Cepeda, H., Pardo, N., Preliminary Conceptual Model of the Paipa Geothermal System, Colombia, Proceedings World Geothermal Congress 2010 Bali, Indonesia, 25-29 April, 2010. [ Links ]
Anderson, M.W., Holmes, S.M., Mann, R., Foran, P., Cundy, C.S., Zeolitisation of diatoms, J. Nanosci. Nanotechnol., 5, 2005, pp. 92-95. [ Links ]
Baccouche, A., Srasra, E., Maaoui, M.E., Preparation of Na-P1 and sodalite octahydrate zeolites from interstratified illitesmectite, Appl. Clay. Sci., 13, 1998, pp. 255-273. [ Links ]
Barrer, R.M., Hydrothermal Chemistry of Zeolites, Academic Press, New York, 1982, 360p. [ Links ]
Barrer, R.M., Beaumont, R., Colella, C., Chemistry of Soil Minerals, Part XIV, Action of Some Basic Solutions on Metakaolinite and Kaolinite, J. Chem. Soc., Dalton Trans., 1974, pp. 934-941. [ Links ]
Barth-Wirsching, U., Holler, H., Experimental studies on zeolite formation conditions, Eur. J. Miner., 1, 1989, pp. 489-506. [ Links ]
Barron, P.F., Frost, R.L., Skjemstad, J.O., Koppi, A.J., Detection of two silicon environments in kaolins by solid-state silicon-29 NMR, Nature, 302, 1983, pp. 49-50. [ Links ]
Boles, J.R., Occurrences of natural zeolites - Present status and future research. In: D. Kallo and H.S. Sherry (eds.), Occurrence, properties and utilization of natural zeolites, Budapest: Akademiai Kiado, 1988, pp. 3-18. [ Links ]
Boukadir, D., Bettahar, N., Derriche, Z., Synthesis of zeolites 4A and HS from natural materials, Ann. Chim. Sci. Mat., 27, 2002, pp. 1-13. [ Links ]
Brathwaite, R.L., Geological and mineralogical characterization of zeolites in lacustrine tuffs, Ngakuru, Taupo Volcanic Zone, New Zealand, Clays Clay Miner., 51 2003, pp. 589-598. [ Links ]
Brathwaite, R.L., Exploration guides for zeolite deposits in lacustrine tuffs, with reference to the Taupo Volcanic Zone, New Zealand, In: R.S. Bowman and S.E. Delap (Eds.), Zeolite '06 - 7th International Conference on the Occurrence, Properties, and Utilization of Natural Zeolites, Socorro, New Mexico USA, 16-21 July 2006. [ Links ]
Breck, D.W., Zeolite Molecular Sieves: Structure, Chemistry and Use, John Wiley, New York, 1974, 313p. [ Links ]
Camprubí, A., Criterios para la exploración minera mediante microtermometría de inclusiones fluidas, Bol. Soc. Geol. Mexicana, 62, 2010, pp. 25-42. [ Links ]
Cañizares, P., Durán, A., Dorado, F., Carmona, M., The role of sodium montmorillonite on bounded zeolite-type catalysts, Appl. Clay. Sci., 16, 2000, pp. 273-287. [ Links ]
Colella, C., Di Palma, G., Aiello, R., Influence of salt addition on the hydrothermal conversion of rhyolitic pumice into zeolite, Mater. Chem. Phys., 12, 1985. pp. 145-155. [ Links ]
Covarrubias, C., Garcia, R., Arriagada, R., Yanez, J., Garland, T., Cr(III) exchange on zeolites obtained from kaolin and natural mordenite, Microporous Mesoporous Mater., 88, 2006, pp. 220-231. [ Links ]
Flaningen, E.M., Khatami, H.A., Szymanski, H.A., Molecular Sieve Zeolites, Adv. Chem. Ser. 101, 16, 1971, pp. 201-227. [ Links ]
Ghiara, M.R., Petti, C., Franco, E., Lonis, R., Luxoro, S., Gnazzo, L., Occurence of clinoptilolite and mordenite in Tertiary calc-alkaline pyroclastites from Sardinia (Italy), Clays Clay Miner., 47, 1999, pp. 319-328. [ Links ]
Gottardi, G., The genesis of zeolites, Eur. J. Miner., 1, 1989, pp. 479-487. [ Links ]
Gottardi, G., Galli, E., Natural Zeolites, Springer-Verlag, Berlin, 1985, 409p. [ Links ]
Hay, R.L., Geologic occurrence of zeolites and some associated minerals. Pure Appl. Chem., 58, 1986, pp. 1339-1342. [ Links ]
Hay, R.L., Sheppard, R.A., Formation of zeolites in open hydrologic system. In: D.L. Bish and D.W. Ming (eds.), Natural zeolites: occurrence, properties, applications, Reviews in Mineralogy and Geochemistry 45, Mineralogical Society of America, Washington, 2001, pp. 261-276. [ Links ]
Hawkins, D.B., Kinetics of glass dissolution and zeolite formation under hydrothermal conditions, Clays Clay Miner., 29, 1981, pp. 331-340. [ Links ]
Hayashi, S., Ueda, T., Hayamizu, K., Akiba, E., NMR study of kaolinite. 1. 29Si, 27Al, and 1H spectra, J. Phys. Chem., 96, 1992, pp. 10922-10928. [ Links ]
Holmes, S.M., Plaisted, R.J., Crow, P., Foran, P., Cundy, C.S., Anderson, M.W., The zeolitisation of diatoms to create hierarchical pore structures, Stud. Surf. Sci. Catal., 135, 2001, pp. 296. [ Links ]
Juan, R., Hernández, S., Andrés, J.M., Ruiz, C., Synthesis of granular zeolytic materials with high cation exchange capacity from agglomerated coal fly ash, Fuel, 86, 2007, pp. 1811-1821. [ Links ]
Kang, S.J., Kazuhiko, E., Modification of different grades of Korean natural zeolites for increasing cation exchange capacity, Appl. Clay. Sci., 12, 1997. pp. 131-144. [ Links ]
Klimkiewicz, R., Drag, E.B., Catalytic activity of carbonaceous deposits in zeolite from halloysite in alcohol conversions, J. Phys. Chem. Solids, 65, 2004, pp. 459-464. [ Links ]
Langella, A., Cappelletti, P., de' Gennaro, R., Zeolites in Closed Hydro-logic Systems, Rev. Miner. Geochem., 45, 2001, pp. 235-260. [ Links ]
Letaief, S., Elbokl, T.A., Detellier,Ch., Reactivity of ionic liquids with kaolinite: Melt intersalation of ethyl pyridinium chloride in an urea-kaolinite pre-intercalate, J. Colloid Interf. Sci., 302, 2006, pp. 254-258. [ Links ]
Manassero, M.J., Zalba, P.E., Andreis, R.R., Morosi, M., Petrology of continental pyroclastic and epiclastic sequences in the Chubut Group [Cretaceous]: Los Altares-Las Plumas area, Chubut, Patagonia Argentina, Rev. Geol. Chile, 27, 2000, pp. 13-26. [ Links ]
Moirou, A., Vaxevanidou, A., Christidis, G.E., Paspaliaris, I. Ion exchange of zeolite Na-Pc with Pb2+, Zn2+ and Ni2+ ions, Clays Clay Miner., 48, 2000, pp. 563-571. [ Links ]
Mumpton, F.A., Mineralogy and geology of natural zeolites: Short Course Notes, Mineral. Soc. Am., 4. 1977, pp. 177-204. [ Links ]
Nivia, A. El Terreno Amaime - Volcánica: Una Provincia Acrecionada de basaltos de Meseta Oceánica, Memorias V Congreso Colombiano de Geología, Bucaramanga, Colombia, Tomo 1, 1-30, 1989. [ Links ]
Nivia, A., Marriner, G.F., Kerr, A.C., Tarney, J. The Quebradagrande Complex: A Lower Cretaceous ensialic marginal basin in the Central Cordillera of the Colombian Andes, J. South Am. Earth Sci., 21, 2006, pp. 423-436. [ Links ]
Pardo, N., Cepeda, H., Jaramillo, J.M., The Paipa volcano, Eastern Cordillera of Colombia, South America: volcanic stratigraphy, Earth Sci. Res. J., 9, 2005, pp. 3-18. [ Links ]
Puerto, R.A., Benito, J.F., Manufacture of zeolite 4A from bauxite, Zeolites, 17, 1996, pp. 315-315. [ Links ]
Ríos, C., Williams, C., Maple, M., Synthesis of zeolites and zeotypes by hydrothermal transformation of kaolinite and metakaolinite, Bistua (Universidad de Pamplona), 5, 2007, pp. 15-26. [ Links ]
Ríos, C.A., Williams, C.D., Synthesis of zeolytic materials from natural clinker: A new alternative for recycling coal combustion by-products, Fuel, 87, 2008, pp. 2482-2492. [ Links ]
Ríos, C.A., Williams, C.D., Roberts, C.L., Removal of heavy metals from acid mine drainage (AMD) using fly ash, natural clinker and synthetic zeolites, J Hazard. Mater., 156, 2008, pp. 23-35. [ Links ]
Ríos, C.A., Williams, C.D., Fullen, M.A., Nucleation and growth history of zeolite LTA as-synthesized from kaolinite by two different methods, Appl. Clay. Sci., 42, 2009a, pp. 446-454. [ Links ]
Ríos, C.A., Williams, C.D., Roberts, C.L., A comparative study of two methods for the synthesis of fly ash-based sodium and potassium type zeolites with potential use in the purification of wastewaters, Fuel, 88, 2009b, pp. 1403-1416. [ Links ]
Ríos, C.A., Williams C.D., Castellanos, O.M., Synthesis of zeolite LTA from thermally treated kaolinite, Revista Facultad de Ingenieria (Universidad de Antioquia), 53, 2010a, pp. 30-41. [ Links ]
Ríos, C.A., Williams C.D., Roberts, C.L., UK fly ash-based zeolites as adsorbents for removal of heavy metals and ammonium from artificially polluted solutions, Ingeniería y Competividad (Universidad del Valle), 12, 2010b, pp. 57-71. [ Links ]
Ríos, C.A., Williams C.D., Hydrothermal transformation of kaolinite in the system K2O-SiO2-Al2O3-H2O, DYNA (Universidad Nacional de Colombia - Medellin), 77, 2010, pp. 55-63. [ Links ]
Ríos, C.A., Williams, C.D., Roberts, C.L., Synthesis and characterization of SOD, CAN and JBW type structures by hydrothermal reaction of kaolinite at 200 oC, DYNA (Universidad Nacional de Colombia -Medellin), 78, 2011, pp. 48-59. [ Links ]
Robert, F., Brommecker, R., Bourne, B.T., Dobak, P.J., McEwan, C.J., Rowe, R.R., Zhou, X., Models and Exploration Methods for Major Gold Deposit Types, In: Proceedings of Exploration 07: Fifth Decennial International Conference on Mineral Exploration edited by B. Milkereit, 2007, pp. 691-711. [ Links ]
Ruiz, R., Blanco, C., Pesquera, C., Gonzalez, F., Benito, I., Lopez, J.L., Zeolitization of a bentonite and its application to the removal of ammonium ion from waste water, Appl. Clay. Sci., 12, 1997, pp. 73-83. [ Links ]
Saikia, B.J., Parthasarathy, G., Sarmah, N.C., Fourier transform infrared spectroscopic estimation of crystallinity in SiO2 based rocks. Bull. Mater. Sci., 31, 2008, pp. 775-779. [ Links ]
Sand, L.B., Zeolite synthesis and crystallization: In Proceedings of the 5th International Conference on Zeolites, Naples, 1980, Rees, L.V.C. (eds.), Heyden, London, I-9. [ Links ]
Sandoval, M.V., Henao, J.A., Ríos, C.A., Williams, C.D., Apperley, D.C., Synthesis and characterization of zeotype ANA framework by hydro-thermal reaction of natural clinker, Fuel, 88, 2009. pp. 272-281. [ Links ]
Shawabkeh, R.A., Synthesis and characterization of activated carboaluminosilicate material from oil shale, Microporous Mesoporous Mater., 75, 2004, pp. 107-114. [ Links ]
Stefánsson, A., Dissolution of primary minerals of basalt in natural waters: I. Calculation of mineral solubilities from 0°C to 350°C, Chem. Geol., 172, 2001, pp. 225-250. [ Links ]
Taylor, M.W., Surdam, R.C., Zeolite reactions in the tuffaceous sediments at Teels Marsh, Nevada, Clays Clay Miner., 29, 1981, pp. 341-352. [ Links ]
Thompson, J. G., Barron, P. F., Further consideration of the 29Si nuclear magnetic resonance spectrum of kaolinite, Clays Clay Miner, 35, 1987, pp. 38-42. [ Links ]
Utada, M., Zeolites in hydrothermally altered rocks. In: D.L. Bish and D.W. Ming (eds.), Natural zeolites: occurrence, properties, applications, Reviews in Mineralogy and Geochemistry 45, Mineralogical Society of America, Washington, 2001, pp. 305-319. [ Links ]
Velandia, F., Cepeda, H., Geología sector sur del municipio de Paipa (Boyacá), Planchas 171 y 191, Instituto Colombiano de Geología y Minería (INGEOMINAS), 2004. [ Links ]
Vitarelli, P., Cavallaro, S., Zipelli, C., Ottana, R., Liquid-solid interaction in granular lipari pumice, Mater. Chem. Phys., 8, 1983, pp. 147-152. [ Links ]
Watanabe, Y., Yamada, H., Tanaka, J., Moriyoshi, Y., Hydrothermal modification of natural zeolites to improve uptake of ammonium ions, J. Chem. Techn. Biotechn., 80, 2005, pp. 376-380. [ Links ]
Weisenberger T., Spürgin S., Zeolites in alkaline rocks of the Kaiserstuhl volcanic complex, SW Germany - new micropobe investigation and their relationship to the host rock, Geol. Belg., 12, 2009, pp. 75-91. [ Links ]