Introduction
Two types of changes characterize puberty in the sexual reproductive system. The first change involves the primary sexual characteristics (changes of the ovaries, uterus, and vagina in girls, and the testes, prostate, and seminal vesicles in boys). The second is the development of secondary sexual characteristics (breast enlargement, appearance of pubic and axillary hair in females; and increase in size of the genitalia, penis, testicles, and scrotum, and the appearance of pubic, axillary and facial hair, and voice timbre change in males) (Chipkevitch, 2001; Meneses & Ocampos, 2008).
The pubertal period is a subsequent neuroendocrine process of the beginning of the pulsatile secretion of gonadotropin-releasing hormone (GnRH) and the reactivation of the hypothalamic pituitary gonadal (Lourenço & Queiroz, 2010). GnRH is guided through blood vessels to the anterior pituitary, where it stimulates the pituitary to secrete two other hormones called gonadotropic hormones the luteinizing Hormone (LH) and the follicle-stimulating hormone (FSH). The gonadotropin-releasing hormone controls both the LH and FSH secretion. The release of GnRH depends on the levels of estrogen and testosterone in the blood and acts through a negative feedback system. High levels of estrogen and testosterone inhibit the GnRH release (Guyton & Hall, 2011; Shier, Butler, & Lewis, 2007; Tortora & Derrickson, 2012).
In women, the LH together with FSH stimulates the secretion of estrogen by the ovaries and causes the release of an oocyte from the ovary, a process known as ovulation. LH also stimulates the corpus luteum formation in the ovary and progesterone secretion by the corpus luteum. In men, LH stimulates the testes to develop and secrete large amounts of testosterone. In the females, blood transports FSH from the anterior pituitary gland to the ovaries, where it stimulates follicular development each month, as well as stimulates ovary cells to secrete female sex hormones. In males, the FSH stimulates the testes to produce sperm. The secretion of testosterone (in men) and estrogen (in women) is what promotes the development of secondary sexual characteristics (Guyton & Hall, 2011; Shier et al., 2007; Tortora & Derrickson, 2012).
Authors Ng, Kumar, Cody, Smith, & Didi (2003) and Zaffuto-Sforza(2005) have indicated that neurological disorders in children could lead to advanced puberty. However, because of the wide variety of bouts studied, there are still only a few studies focused on the direct relation between Cerebral Palsy and precocious puberty. With this in mind, this study aimed to evaluate the pubertal development of children diagnosed with Cerebral Palsy and identify the gender with the highest incidence of advances in the maturation of primary and secondary sexual characteristics. A study like this becomes necessary to analyze how Cerebral Palsy can affect the changes in puberty by gender.
Understanding the neuroendocrine processes that bring about the beginning of puberty and knowing the science of individual staging of puberty allows healthcare professionals to guide parents and relatives on the necessary behaviors to deal with the conditions related to precocious puberty, such as short stature (early suspension of growth), and the early changes in body and behavior. These changes make these children drive themselves away from their group of friends of the same age, and by doing this, they start to have psychological problems that require more attention and often demand therapeutic help.
Methods
This study was duly submitted to the Ethics in Research Committee (CEP) of the Centro Universitario Campos de Andrade (UNIANDRADE) and granted certificate for Ethics Assessment (CAEE) n° 23622113.0.0000.5218.
Twenty-five Caucasian children were assessed, 11 girls and 14 boys, between the ages of five and ten, all of them with a clinical diagnosis of Cerebral Palsy. The children's parents or guardians read, accepted and signed an Informed Consent form, which explained the evaluation process of the individuals and the procedures to be performed. All of the children are patients of the Victoria Neurological Rehabilitation Center.
The data from the questionnaire to parents and guardians regarding the pubertal development of the children was collated; subsequently, the children’s stage of sexual maturation was evaluated and classified.
Marshall and Tanner established sexual maturation stages for boys (Marshall & Tanner, 1970) and girls (Marshall & Tanner, 1969). Physical examination can assess these stages, and range from Stage 1 (child) to 5 (adult), considering for females the size, shape, and characteristics of the breasts (M) and also the quantity, distribution, and characteristics of pubic hair (P). For males, the size, shape and characteristics of male genitals (G) and also quantity, characteristics, and distribution of pubic hair (P). This scale (Table 1) is widely used for classification of staging puberty in children and teenagers. Thus, the Tanner classification was used to classify the staging puberty, with confidentiality guaranteed and the rights to copy consent at any time.
A male evaluator was trained to assess the boys, and a female, to assess the girls, in order to avoid possible embarrassment by the children and have a better acceptance by parents or guardians.
This study did have a control group due to non-consent of the parents/guardians of children considered typical (without PC), which did not happen with the parents/guardians of atypical children, once they realized and understood the importance and benefits of this study. However, the findings of Colli (Colli, 1986) were used as a reference because these authors published a study with a large sample of healthy children living in Santo André (SP).
The Shapiro-Wilk test was used to check the distribution of variables. To characterize the sample’s central tendency we used mean and for dispersion standard deviation. To analyze differences between groups we used the Student t-test for independent samples. All calculations were carried out considering a significance level of 95% (p <0.05).
Results
The results of the evaluation of the children’s’ maturation using the Tanner scale are presented in Table 2.
Table 2 Tanner Scale Sample Distribution of 25 children from five to ten years old (11 girls and 14 boys), all of them with a clinical diagnosis of Cerebral Palsy
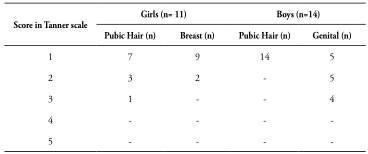
Three girls were observed (1 = P2M1; 2 = Tanner P2M2) and nine boys (5 = P1G2; 4 = Tanner P1G3), all with precociously matured secondary sexual characteristics.
Only two female patients had Tanner = M2/P2 simultaneously, both five years old on the day of collection. All other findings were isolated, one ofTanner = 1 and the other items ofTanner > 1. Two females presented isolated pubarche (one 7-years and 3-months old and the other 9-years and 6-months old on the date of collection). Only the youngest presented precocious maturation (before 8-years old).
In boys, the age of children with G = 2 and G = 3 was 6.2 ± 0.0 and 7.6 ± 0.0 years, respectively. Table 3 shows the statistical comparison between genders for the evaluation variables of maturation, according to Tanner scale.
Table 3 Comparison of pubertal stage between genders by Student t-test for independent samples, from 25 children with a clinical diagnosis of Cerebral Palsy

A statistically significant difference is observed between the development of genitalia in boys and breast in girls, in which the boys have a higher rate of maturation of genitals than the girls of breasts (thelarche). On the other hand, girls are more likely to develop hair than the boys (pubarche).
The results of this study indicate an average chronological age for girls classified as P2 = 5.9 years, P3 = 9.5 years, and as M2 = 5.2 years; for the boys, the average age for G = 2 was 6.2 years old and for G3 was 7.6 years old. To establish an average chronological age for each score of the Tanner scale, in Brazilian children, Colli (Colli, 1986) evaluated 2880 children between 10 and 19 years (1410 girls and 1470 boys), from Santo André city (Sâo Paulo), with no apparent neurological disease. Table 4 shows the findings by Colli (Colli, 1986).
Table 4 Mean age observed for each score of the Tanner scale, for typical children, according to Colli and for children with cerebral palsy evaluated in this study (2015)

* Significant statistically difference between samples (Colli study (11) and This Study) of the same category (p <0.001).
Source: Colli (1986).
Discussion
Comparing the results with the reference of typical children proposed by Colli (Colli, 1986), one can observe an advanced maturation of sexual characteristics in the children with CP evaluated in this study. In another study (Oliveira et al., 1994), involving Brazilian children, which evaluated 15 children who developed secondary sexual characteristics -girls before eight and boys before nine years of age- correlating them with important neurological findings. From these 15 children, seven cases of actual precocious puberty were diagnosed, as well as four cases of neurogenic etiology, two of precocious thelarche, and five of precocious pubarche. In our study, we found one case of precocious pubarche and two cases of pubarche associated with precocious thelarche.
The physiological explanation for this chronological advance of puberty may be linked to the premature release of the gonadotropic hormones secondary to hypothalamic-pituitary activation (Macedo, Cukier, Mendonca, Latronico, & Brito, 2014). This activation may be caused by hydrocephalus, brain trauma, perinatal anoxia, chemotherapy or radiotherapy CNS seizure syndromes, infections of the CNS or some pituitary abnormalities (e.g., hormone-secreting tumor), or by an abnormality in the hypothalamus (hamartoma, astrocytoma, neurofibromas, hypothalamic maturation secondary to early exposure to endogenous or exogenous sex steroids) (Macedo et al., 2014). This premature release of secondary gonadotropic hormones in hypothalamic-pituitary activation could have occurred in the sample studied in three girls (1 = P2M1; 2 = Tanner P2M2) and nine boys (5 = P1G2; 4 = Tanner P1G3) with precociously matured secondary sexual characters.
Carvalho et al. (2007) conducted a retrospective study that reviewed medical records of 58 patients (all female and without neurological affections) with suspected precocious puberty. It was found that of the 58 cases, 28 were diagnosed with central precocious puberty, one with precocious pseudopuberty, 10 with precocious thelarche and 19 with precocious pubarche. Other interesting results were the average age of the appearance of premature pubarche (5.2 ± 1.5 years) and precocious thelarche (2.2 ± 2.4 years). These results are consistent with the ones in this study as it refers to precocious pubarche, whose average age of children was 5.9 years. However, our findings to the mean age of children with precocious thelarche were 5.21 years. In the two cases presented in our results, pubarche was associated with precocious thelarche.
In pseudo precocious puberty, cited in the study by Carvalho et al. (2007), there is an autonomous secretion of high-levels of androgens or estrogens by the gonads, regardless of GnRH. These hormones do not induce the maturation of sex glands; it only gives the child an adult appearance. The most common causes are congenital adrenal hyperplasia virilization, and androgen-secreting tumors (in males), and estrogen-secreting tumors (in females), as well as interstitial cell tumors (testicular), tumors of granulosa cells (ovarian), and inadvertent exposure to exogenous estrogens (Macedo et al., 2014).
The precocious thelarche cases (early breast development in women) that happen without pubarche (onset of pubic hair) and accelerated bone maturation, do not require treatment. It is suggested that there exists a continuing condition of puberty, ranging from early idiopathic thelarche to actual precocious puberty (Bizzarri et al., 2013). Hence, the two 5-year old girls of this study showing a Tanner P2M2 score, which were referred for a more detailed study to initiate appropriate treatment of this situation.
Studies covering topics like this (puberty), are usually considered a “taboo” in the society, making it difficult for the researcher to obtain a significant consent from most of the parents/guardians, due to their apprehension to allow their children to participate in the study. Other factors that limited this study were the unfeasibility of conducting hormone measurements to confirm the frames evaluated by the Tanner scale because of the scarcity of resources and specific publications related to the direct relationship between bouts of CNS and pubertal development of children.
Conclusion
The results of this study reinforce the hypothesis that there is a relationship between neurological disorders and the advance in maturation of secondary sexual characteristics, as our findings suggest the involvement of this maturation before it is considered an average age in children. However, to have a better understanding of this relation, studies with hormone dosage and characterization of neurological affections are necessary.















