INTRODUCTION
The increasing demand for water resources in recent decades has led to the search for more water-efficient techniques, as well as the rational use of water that is not desirable for drinking. The agricultural production sector puts the highest pressure on water resources. According to Chartzoulakis and Bertaki (2015), of the total annual volume of estimated water diversion worldwide, agricultural production currently uses about 70%, mainly for irrigation.
In this context, according to some authors (Dias et al., 2010; Cosme et al., 2011; Santos Júnior et al., 2015), hydroponic agriculture is a strong ally as a mitigating technology against the quantitative and qualitative depletion scenario of water resources for the following reasons. Firstly, this approach favors water-efficient applications in agriculture.
The other reason involves the use of water that is considered marginal, such as brackish water. Alves et al. (2011) reported that, as hydroponic cultivation frequently assumes a matric potential equal to zero because of the saturation of plants, the crop response to salinity increases, as compared to the conventional cultivation system. Moreover, the authors pointed out that the drainage and irrigation system (Nutrient Film Technique) enables the appropriate use of mineral salts at the end of production. Numerous studies have shown (Paulus et al., 2010; Soares et al., 2010; Alves et al., 2011; Maciel et al., 2012; Sarmento et al., 2014) promising results using brackish water in the hydroponic cultivation of vegetables and some ornamental plants.
Moderately sensitive to salinity (Ayers and West-cot, 1985; Kurunc et al., 2011; Maia et al, 2017), bell peppers (Capsicum annuum L.) are one of the most widespread vegetables that are consumed fresh in Brazil. According to Albuquerque et al. (2011; 2012), the sweet pepper is among the ten most economically important vegetables in the domestic market. This is due to its fast vegetative growth and impact on production that provides an immediate financial return. Thus, it is widely cultivated by small and medium-sized farms. Sweet peppers originate from Central and South America and can be produced year around in warm climates (Monteiro et al., 2009).
Accordingly, this study aimed to evaluate the effect of brackish water on sweet pepper production under the NFT system at different electrical conductivity levels.
MATERIALS AND METHODS
This experiment was conducted in a greenhouse in the municipality of Cruz das Almas-BA, Brazil (12°40'19" and 39°06'23" W; 220 m a.s.l.). The greenhouse structure used galvanized steel pipe covered with two arches and protected on the sides by a clarite type screen. The plastic film (anti UV, 150 /xm thickness) was used to cover the roof and a thermal reflective blanket (Aluminet 50%, Ginegar Polysack, Leme-RJ, Brazil) was used inside, 14 m wide X 32 m long X 4 m high, with a maximum height of 5.5 m. In order to improve the phytosanitary conditions of the greenhouse and extend the work life of the electric pumps (Brastemp, São Paulo-SP, Brazil), the floor was covered with a Bidim geotextile drainage blanket (Fibratex, Indaiatuba-SP, Brazil).
Sweet pepper seeds (Capsicum annuum L.) cv. Rubi Giant were seeded in a 50 mL plastic cups containing a layer of coconut fiber substrate and another layer of vermiculite at a 2:1 ratio (v/v) on November 28, 2013. Four seeds were placed in each cup. Thinning was done 15 days after sowing, leaving only one plant per cup.
During the experiment, the air temperature and relative humidity were monitored inside the greenhouse using a Thermo-Hygrometer sensor (Dostmann electronic GmbH, Wertheim, Germany) (Figs. 1A and B). The temperature was also measured with thermocouples (Copper/Constantan) in two different reservoirs of the nutrient solution. The sensors were connected to a data logger (model CR 1000, Campbell Scientific, São o Paulo-SP, Brazil) and the averages were recorded every 30 min.
The experiment design consisted of 35 experiment units. Each plot represented an independent NFT system (Nutrient Film Technique) containing a plastic reservoir with a 60 L capacity for the nutrient solution and one electric circulating pump. The structure consisted of hydroponic profiles of polypropylene, with a commercial diameter of 100 mm, anti-UV additives, 3 m length and five holes with a 0.05 m radius, at a spacing of 0.30 m between the profiles.
The profiles were installed in pairs at a height of 1 m from the ground, with four points of support and a gradient of 4%. The spacing between the profiles was 0.5 m and a 0.9 m wide corridor was left between pairs of plots to facilitate transit and operability (Fig. 2).

Figure 2 Illustration of hydroponic system NFT with automatic supply reservoir (adapted from Soares, 2007). 1. Hydroponic profile; 2. PVC conduit leading the solution to the injection system; 3. Conductive PVC tube of the solution not injected to the reservoir; 4. Circulation pump; 5. Float cock; 6. Nutrient solution reservoir; 7. Graduated transparent hose; 8. Automatic supply system.
The nutrient solution was conducted from the reservoir to the upper bench reaches through 0.02 m diameter PVC pipes, through which the solution was injected into the hydroponic profile with extension hoses. In the lower part of the profile, a cap was installed with a hose in order to return the excess nutrient solution to the tank. The surplus that was not injected into the profile was returned to the reservoir with a PVC pipe, where a 90° elbow was installed to improve the aeration of the nutrient solution.
Furthermore, each plot had an automatic supply system built with a solid section of PVC pipe and a nominal diameter of 0.2 m. This type of system allows automatic water output to the nutrient solution tank through a float-tap and automatic restoration of water consumption during evaporation. The system had a graduated scale fixed with a transparent hose, allowing for calculation of the evapotranspiration volume per plant at any time with equation 1.
where:
VETC |
: evapotranspiration volume (mL per plant and day); |
Li and Lf |
: initial and final water level readings, respectively, in the automatic supply reservoir, (m); |
D |
: the inner diameter of the supply tank (m); |
ΔT |
: the time interval between daily readings; |
n |
: the number of plants in the hydroponic profile in the time interval ΔT |
The experiment design was randomized into blocks with seven treatments and five replicates. The treatments comprised the electrical conductivity of the water (ECw = 0.29; 1.39; 2.75; 4.49; 5.90; 6.76 and 7.09 dS m-1) used for both the nutrient solution preparation and replenishment of crop evapotranspiration.
The nutrient solution used to cultivate hydroponic pepper was similar to that recommended by Benoit et al. (1987). Table 1 shows the composition of the nutrient solution used in the experiment.
The water (ECw = 0.29 dS m-1) came from the municipal supply (tap water), and the brackish water (EC = 1.39; 2.75; 4.49; 5.90; 6.76 and 7.09 dS m-1) was obtained by adding NaCl to the tap water. Therefore, the final ECsol in the nutrient solution preparation was 2.11; 4.13; 5.10; 6.19; 7.26; 8.03 and 8.54 dS m-1, respectively.
The nutrient solution was pumped from the reservoir to the upper bench reaches with an electric circulating pump, which was triggered with a digital programmable timer. It ran at time intervals of 15 min on and 10 min off during the periods from 06:00 to 11:00 am and from 02:00 to 07:00 pm. From 11:00 am to 14:00 pm, the irrigation was uninterrupted. In addition, there was irrigation for 15 min at 9:00 pm, 11:00 pm and 2:00 am.
The plants were manually irrigated with water from the local supply (ECw = 0.29 dS m-1) until transplant to the hydroponic profiles. The experiment began on December 21, 2013 (23 d after sowing, das) by transplanting nine seedlings to each hydroponic plot when they presented two pairs of true leaves. During the experiment, the sweet peppers were vertically staked using polythene strips attached to horizontal and parallel strands of wire positioned 2.20 m from the ground. An application of Trichodel biofungicide (0.16 mL L-1 of nutrient solution) was done during the experiment and two applications of nim (Azadirachta indica) leaf extract (10% v/v) were done within 10 d. The electric conductivity and pH of the nutrient solution were evaluated every two days. The ECsol was monitored with a bench conductivity meter, and, since the nutrient solution was not replaced, it was necessary to replenish the nutrients consumed at 33, 35, 42 and 44 d after transplanting (dat), as shown in table 2. The pH of the nutrient solution was always adjusted when necessary with 20% phosphoric acid in order to maintain the nutrient solution in the range of 5.5 to 6.5 pH. as proposed by Furlani et al. (1999).
The harvest occurred on February 28, 2014, that is 69 dat or 92 das. The parameters: fruit diameter, fruit length, number of fruits per plant, mean fruit weight, total weight of fruits per plant and fruit length/diameter ratio were measured on that day.
The data were submitted to the normality assumption (Shapiro-Wilk) and homoscedasticity test (Bartlett test). The data were submitted to analysis of variance (ANOVA) and, when significant differences were verified by the F test at 5%, the means were submitted to regression analysis in order to verify the optimal concentration for each variable through the first derivative of the estimators 00 and P1. The significance of the models, the biological meaning and the coefficient of determination (R 2 ) were considered when choosing the equations. The analysis was performed using the statistical program Sisvar® (Ferreira, 2011).
RESULTS AND DISCUSSION
The initial salinity of the treatments was variable because of the fertilizer salts added to the water for sweet pepper productivity and NaCl usage for increasing the electrical conductivity (Fig. 3). The initial EC value in the water supply was 0.29 dS m-1 and, in the brackish waters, it was 1.39; 2.75; 4.49; 5.90 and 7.09 dS m-1. Thus, the EC after the addition of the nutrient solution (ECsol) was 2.11; 4.13; 5.10; 6.19; 7.26 and 8.54 dS m-1.
It was observed that EC was reduced in the treatment without brackish water (0.29 dS m-1) during the experiment. The other treatments increased their EC until the 59th day of evaluation with similar results. However, after 61 dat, the treatments 5.90, 6.76, and 7.09 dS m-1 overlapped (Fig. 3).

Figure 3 Electrical conductivity of nutrient solution after salt addition to the NFT hydroponic system.
The electric conductivity (EC) in the treatment without brackish water was reduced during the experiment because the plants consumed the nutrients as a result of the absence of a corresponding ion inflow by water replacement even though the nutrient replacement occurred at 33; 35; 42 and 44 dat. Similar results were found in growing ornamental sunflowers and lettuce in the NFT hydroponic system by Maciel et al. (2012) and Alves et al. (2011), respectively. Moreover, according to these authors, this small reduction in the salinity resulted from a nutrient intake that was greater than the contribution of salts dissolved in this treatment.
The increased salinity from 61 dat in the treatments 5.90, 6.76, and 7.09 dS m-1 was due to ion accumulation not absorbed by the sweet pepper plants. This could be because the evaporative water loss was carried out with salt water at each EC in the treatments and linked to increasing water consumption of the bell pepper during the growth stages (Figs. 3 and 5).

Figure 4 Number of sweet pepper fruits per plant grown in nutrient solution with different salinity levels in the NFT hydroponic system.
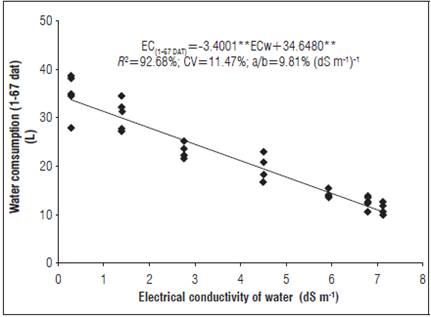
Figure 5 Water consumption of sweet pepper grown in nutrient solution with different salinity levels in the NFT hydroponic system.
As seen in figure 4, only 22.6% of the dependent variable could be explained by the covariates in the model. However, the number of fruits per plant showed a small decrease as salinity increased in the nutrient solution (Fig. 4). Chartzoulakis and Klapaki (2000) had similar results in their research. They found a drastic reduction in the number of fruits when the salinity level exceeded 7.0 dS m-1. Furthermore, several authors have observed linear reductions in plant dry matter production associated with increasing salinity of the environment and have concluded that this is responsible for reducing the number of fruits and fruit weight per pepper plant (Navarro et al., 2010).
The decrease in these parameters reflects the result of negative osmotic pressure in the nutrient solution because of salt stress. Therefore, the plants responded physiologically by closing stomata, inhibiting water absorption (Fig. 6) and inducing cell dehydration, toxic salt, and, finally, senescence (Cosme et al., 2011). Moreover, plant growth in saline solutions may be affected by an inverse correlation between K and Na accumulation in the leaf tissue, related to the competition in the plasma membrane of these ions for absorption and transport sites. Thus, K concentrations in leaf tissue decrease by increasing concentrations of Na because of the antagonism between these elements (Soares et al., 2016).

Figure 6 Average fruit diameter of sweet pepper grown in nutrient solution with different salinity levels in the NFT hydroponic system.
Potassium is vital in the water-plant relationship, helps maintain the internal pressure of plant cells and produces more succulent fruits. The root system in potassium deficient plants is poorly developed, which impairs the absorption of water and nutrients. Potassium also plays an important role in enzymatic reactions, carbohydrate and protein metabolism, sugar and starch translocation, water-plant relationships, and cell division (Marschner, 2012).
The nitrogen levels in the solution reduced the effect of salinity on the plant dry weight. This result occurred with irregular concentrations in plants grown in the N salinity of 3.4 dS m-1 and in plants at suitable levels of N with a salinity of 6.2 dS m-1 (Semiz et al., 2014), i.e. the data dispersion in figure 5 may be related to the nutrient content in the NFT system. Water absorption, essential for cell growth, was inhibited by the low potential of water around the roots because of the reduction of the osmotic potential in the saline conditions, causing a water deficit and reduction of the evapotranspiration replenishment when using both the brackish and tap water (Soares et al., 2015).
Fruit width and length are morphological traits that, generally, describe fruit size and appearance in bell peppers. As seen in figures 6 and 7, there were reductions of 26.6 and 25.6% in the fruit diameter and length, as well as an electrical conductivity in the solution of 7.09 dS m-1 when compared to the control treatment (0.29 dS m-1). The other treatments (1.39; 2.75; 4.49 and 5.90 dS m-1) had reductions in the fruit diameter of approximately 8.6; 13.7; 18.9 and 24.0% and in the fruit length of approximately 5.6; 12.4; 20.1 and 24.0%, respectively.

Figure 7 Average fruit length of sweet pepper grown in nutrient solution with different salinity levels in the FT hydroponic system.
These results were reflected in the average fruit weight per plant (Fig. 8), as well as the number of fruits per plant (Fig. 4), which were affected by the increasing salinity. According to Navarro et al. (2010), salinity reduces both fruit size and number of fruits or only fruit size. This corroborates our results. A study conducted by Arruda et al. (2011) observed a reduction in weight of marketable fruits of 6.67% for each unit of increased electrical conductivity.

Figure 8 Average fruit weight for sweet pepper fruit grown in nutrient solution with different salinity levels in the NFT hydroponic system.
This difference in these results is explained by the initial electrical conductivity of each experiment (2.6 and 0.29 dS m-1, respectively), an almost 9-fold difference. However, De Pascale et al. (2000) observed that yield (measured by individual fruit weight and number of fruits per plant) was only significantly reduced by salt stress when NaCl concentrations were higher than 0.25% and electrical conductivity of the soil was greater than 4 dS soil m-1 in the root zone.
The EC was 2.8 dS m-1 and 53% between the higher conductivity (2.6 dS m-1) and the control treatment (12.2 dS m-1). Figure 7 shows a reduction of approximately 65.4% in fruit weight between the control treatment (0.29 dS m-1) and the treatment with higher conductivity (7.09 dS m-1).
The behavior of the variables length, diameter and average weight (Figs. 6-8) was similar to that obtained by Leonardo et al. (2007), who attributed this physiological crop response to two factors: a negative interaction between the levels of Ca and K in the plant and low availability of Ca for plants in a salt stress environment.



















