Introduction
Cisplatin-based CT is the treatment of choice for advanced NSGCT, with a ten-year OS of 90%1. A resid ual mass (RM) in NSGCT after CT is defined as a mass > 1 cm in greatest diameter2. The preferred treatment for RM is retroperitoneal lymph node dissection (RPLND) which should be performed 5-6 weeks after the last cycle of cisplatin-based CT (first line), with a cure rate greater than 80%2,3. The most common his tology after pathological examination in an RM is necro sis in 40-50%, followed by teratoma in up to 40% and 10-15% viable tumor4, with a recurrence rate for teratoma and viable disease of 6-39% at 2 years4. Furthermore, viable disease has the worst prognosis of them all, with a 4-year PFS and OS of 57.8% and 66.8%, respectively5. Teratoma is an unpredictable tumor that has the capacity of local growth or malignant somatic transformation to sarcoma or carcinoma5. The aim of our study is to describe the oncological out comes in patients with CS II and CS III NSGCT with an RM after primary or secondary CT.
Materials and methods
From 2007 to 2020 a total of 188 patients were diag nosed with NSGCT in our institution. Of these, 60 men fulfilled the inclusion criteria and were analyzed. We included patients diagnosed with NSGCT clinical Stage II or III, who had received primary or secondary line systemic CT, had negative tumor markers after CT, and had an RM > 1 cm in the greatest diameter. We excluded patients who had desperate RPLND and extra-abdominal residual masses.
The main objective was to evaluate the PFS in patients with NSGCT CS II and III with RM after CT. The secondary objectives were to describe the type of treatment of patients with NSGCT and RM after CT, evaluate the OS between patients with NSGCT clinical Stage II and III, evaluate the PFS and OS according to the International Germ Cell Cancer Collaborative Group (IGCCCG) risk classification, describe the PFS and OS between the types of treatment for the RM, characterize the histology of RM that underwent RPLND and describe the OS according to the histology found after RPLND.
There were two types of treatments, bilateral RPLND, and observation, the aim of surgery was to make a complete resection in every case. Observation was offered to one patient who had an unresectable mass, and patients who were offered a follow-up based on images and tumor markers, but didn't come back to follow-up, we believe because of the limited health access in our country. In our analysis, we included the pathology report of both RPLND and biopsy when it was performed. We define complete resection as free microscopic surgical margins.
Masses greater than 1 cm in diameter were consid ered RM. PFS was defined as the time of diagnosis of RM after CT to disease progression. Progression was defined as mass growth when no surgical treatment was offered and as new evidence of retroperitoneal mass in images when surgery was performed, an increase of serum tumor markers, new metastatic lesions, and death. OS was defined as the time of diag nosis of RM after CT to death of any cause.
The statistical analysis was performed using STATA. Kaplan Meier curves were used to evaluate PFS and OS. A p < 0.05 was considered statistically significant and hazard ratios with 95% CIs were reported for our cox regression model.
Results
A total of 60 men were included in our analysis, the median follow-up time was 33 months. The median age at diagnosis of the RM was 25, 5 years, 53.3% of patients had a pT1 disease, 65% were N3, 55% of patients had an S1 stage and 63.3% were M0 at the initial diagnosis. Half of the cohort included was CS II and the other half was CS III. Most of the patients (63.3%) had a good IGCCCG prognosis group and 15% had a poor IGCCCG prognosis group. The most of patients received first-line CT with bleomycin, etoposide, and cisplatin (BEP). The median size of the mass before and after CT was 50 mm and 58 mm respec tively, as shown in table 1.
Table 1 Baseline characteristics
| Variable | n = 60 (%) Value |
|---|---|
| Age, years, Median (IQR) | 25.5 (21-29) |
| Primary tumor | |
| Gonadal | 58 (96.6) |
| Extra-Gonadal | 2 (3.4) |
| Follow-up, mo, Median (IQR) | 33 (17-74) |
| pT, n (%) | |
| pTX | 3 (5) |
| pTIS | 2 (3.3) |
| pT1 | 32 (53.3) |
| pT2 | 19 (31.7) |
| pT3 | 4 (6.7) |
| cN, n (%) | |
| N1 | 5 (8.3) |
| N2 | 16 (26.7) |
| N3 | 39 (65) |
| cM, n (%) | |
| Mx | 1 (1.7) |
| M0 | 38 (63.3) |
| M1A | 17 (28.3) |
| M1B | 4 (6.7) |
| S stage, n (%) | |
| Sx | 4 (6,7) |
| S0 | 6 (10) |
| S1 | 33 (55) |
| S2 | 10 (16.7) |
| S3 | 7 (11.7) |
| Clinical stage, n (%) | |
| II | 30 (50) |
| IIA | 4 (6,7) |
| IIB | 11 (18.3) |
| IIC | 16 (26.7) |
| III | 30 (50) |
| IIIA | 11 (18.3) |
| IIIB | 8 (13,3) |
| IIIC | 10 (16.7) |
| IGCCCG Risk, n (%) | |
| Good | 38 (63,3) |
| Intermediate | 13 (21,7) |
| Poor | 9 (15) |
| Pre-chemotherapy mass size, mm, median (IQR) | 51 (33-75.75) |
| First-line chemotherapy, n (%) | |
| BEP | 57 (95) |
| EP | 1 (1.7) |
| VIP | 2 (3.3) |
| Second line chemotherapy, n (%) | 5 (8.3) |
| Post-chemotherapy mass size, mm, median (IQR) | 58 (30-78) |
| Treatment, n (%) | |
| RPLND | 54 (90) |
| Observation | 6 (10) |
| Resection, n (%) | |
| Complete | 51 (94.4) |
| Incomplete | 3 (5.6) |
| Pathology, n (%) | |
| Teratoma | 39 (73.6) |
| Fibrosis | 12 (22.6) |
| Viable tumor | 2 (3.8) |
| Resected lymph nodes, median (IQR) | 21 (12-32) |
| Positive lymph nodes, median (IQR) | 0 (0-2) |
Of all patients included, 90% of them underwent bilat eral RPLND, and 94.4% had complete surgical resec tion. The most common pathological finding was teratoma in 73.6% of patients, followed by fibrosis in 22.6% and viable tumor in 3.8% of patients shown in table 1. Progression was seen in 15% of patients and 13.3% died of any cause in the entire cohort.
Three patients had an incomplete surgical mass resection, during follow-up no one of them had onco logical progression and one of them received sec ond-line CT.
The PFS was longer in patients with NSGCT CS II s CS III with a p = 0,02 being a statistically significant finding. The median time to progression for CS II was 131 months and NR for CS III due to the small sample of patients shown in figure 1A. On the other hand, there was no difference between the IGCCCG risk group and PFS.
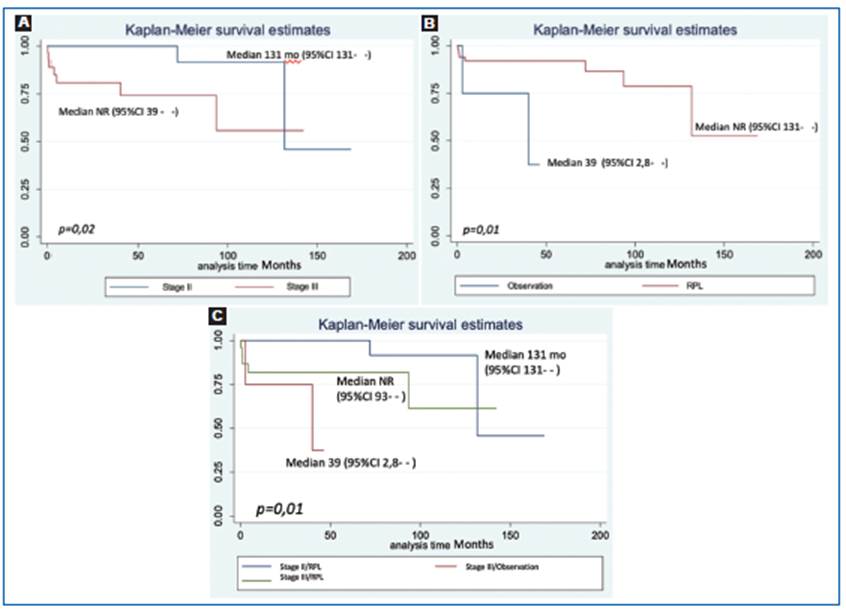
Figure 1 PFS Figure 1: Kaplan Meier estimates of progression-free survival (PFS) analysis. A: PFS differences between clinical Stage II and III (p = 0.02). B: PFS analysis between type of treatment (observation vs RPL) (p = 0.01). C: PFS analysis between clinical stage and type of treatment (p = 0.01).
When comparing the types of treatments, we found that patients left in observation had an increased risk for progression with a median of 39 months (95%CI 2.8- -) vs patients who had RPLND with a median NR (95%CI 131- -) p = 0,01 being this a significant finding shown in figure 1B. Likewise, patients with CS III dis ease that were left in observation had an increased risk for progression with a median of 39 months (95%CI 2.8- -) vs CS II patients who had RPLND with a median of 131 months (95%CI 131- -) p = 0.01 shown in figure 1C.
On the other hand, OS was longer in patients with CS II when compared to CS III (p = 0.053) shown in figure 2A. Furthermore, there was a longer OS in patients with IGCCCGC good prognostic group and worst OS in patients with IGCCCGC intermediate prog nostic group (p = 0.059). When comparing treatment modalities, OS was longer in patients who underwent RPLD with a median NR (95%CI 131- -) vs patients in observation with a median of 40 months (95%CI2.8- -) (p = 0.01) (Fig. 2B), on the other hand, CS III patients that were in observation had worst OS with a median of 40 months (95%CI 2.8- -) vs CS II patients who had RPL with a median NR (p = 0.019) (Fig. 2C).

Figure 2 A: OS difference between CS II and CS III (p = 0.05). B: OS differences between types of treatment (p = 0.01). C: OS analysis between clinical stage and type of treatment (p = 0.01). D: OS and pathology of residual mass (p = 0.02).
When comparing OS and pathology results, we found that patients who had viable tumors had a median OS of 11 months vs. 131 months in patients with teratoma and a median NR in fibrosis (p = 0.02) (Fig. 2D).
In the univariate analysis, we found that patients with CS III disease is a predictor of worst PFS HR 5,1 (95% CI 1-25; p = 0,04). Similarly, the intermediate IGCCG risk group is predictive of the worst OS HR 6,8 (95%CI 1,15-40.7; p = 0.034). On the other hand, patients who had RPLD have better PFS when compared to obser vation, HR 0,1 (95% CI 0.02-0.8; p = 0.03) shown in table 2.
In our cohort, 8.3% of patients had a PET/CT FDG before surgery, and 80% of the patients had a positive result. All the patients with positive results had surgery; the pathology result was 50% for teratoma, 25% for viable tumor, and 25% for fibrosis. One patient had a negative scan and he also had surgery, with the pathol ogy report being positive for fibrosis.
Discussion
RPLND for residual retroperitoneal mass after CT is the mainstay of treatment with a cure rate of >80%3. Complete resection with negative surgical margins is the goal of treatment, otherwise this is associated with an increased risk of recurrence and death5,6. Progression after RPLND is a major problem, with a rate of viable tumor in 9-31% of patients in pathology reports after RPLND, indicating the need of additional treatment5,7-9. On the other hand, teratoma is known to be chemoresistant and has the potential for malignant transformation and RPLND is curative in this setting10,11. There have been described important survival factors in patients with viable tumors5,12,13, and recommended to remove all residual lesions larger than 1 cm in patients with NSGCT14-16.
Luz et al. describe their experience in patients with NSGCT who had RPL, they included 73 patients, and found teratoma in 41.1% of patients with RM, followed by fibrosis in 37% and viable tumor in 21.9%, the recur rence rate was of 9,6%. Compared to our study, we also had teratoma (70%) as the most common pathol ogy in patients with RM, followed by fibrosis and viable tumors. On the other hand, the recurrence rate in our study was greater (15%)3.
On the other hand, Napier et al. evaluated the out comes of patients with NSGCT with RM after QT and negative tumor markers, with a median follow-up of 66 months. They included 76 patients of whom 48 had surgery and 28 patients were observed. Above 90% and 80% of patients were alive, and 70% and 80% were disease-free after surgery and observation, respectively, these findings were not statistically signif icant (p = 0.05 and p = 0.3, respectively) (12. In our cohort, patients who were observed had an increased risk of progression compared to patients having RPL (p = 0.01) as well as shorter OS in patients observed compared to surgical intervention (p = 0.01).
Altan et al. evaluated the clinical characteristics of patients with NSGCT and viable tumors after RPLND, they found a PFS at 5 years of 57.8% and OS at 5 years of 66.8%5. In our cohort, only 3.8% of patients had viable tumors and the median OS was 11 months in this group of patients.
There is scarce data of the usefulness of the PET/CT FDG in NSGCT with inconclusive results12,17-19, Oechsle et al. conducted a multicenter study, where they found PET can predict viable tumors in 56% of cases, and has an overall sensitivity and specificity of 70% and 48, respectively14. In our study, 80% of PET's were positive with teratoma being the most common finding.
There is a lack of information on testicular cancer in the Colombian population due to the lack of support for research and the absence of specialized care programs for this pathology. The National Cancer Institute is the reference center in Colombia with the largest number of patients undergoing retroperitoneal lymphadenectomy, being a reference center with more than 50 years of experience in directed care protocols. This is the first study that provides a histological evaluation directly on the lymphadenectomy product. The main limitation in our study is the retrospective nature, which implies observation and selection bias. On the other hand, the small sample of patients and the follow-up is a major limitation, given that some patients were able to con sult other health centers, which impacts directly on the results. We consider it is necessary to keep expanding the data on this pathology with prospective studies.
Conclusion
In our study, NSGCT CS III had the worst PFS, and OS as expected. Interestingly patients with viable tumors after RPLND had worse OS than patients with Teratoma and may benefit from consolidation chemo therapy. RPLND continues to be the treatment of choice to patients with residual tumor masses after CT and negative tumor markers.















