Introduction
Estrogens and androgens are steroid hormones present in both men and women, regulated by the hypothalamic-pituitary-gonadal (HPG) axis. The secretion of gonadotropin-releasing hormone (GnRH) by the hypothalamus stimulates the production of luteinizing hormone (LH) and follicle-stimulating hormone (FSH) by the anterior pituitary gland, which ultimately leads to the production of testosterone (T). Testosterone acts as an androgen or can be a substrate for estrogen synthesis1. While typically considered a female hormone, estrogen also influences male function. Aromatase catalyzes estrogen synthesis through the aromatization of testosterone, with approximately 20% produced in Leydig cells, with the remaining percentage being produced in tissues such as adipose, brain, skin, liver, and bone tissue2.
Kisspeptin neurons located in the hypothalamus generate excitatory stimuli on the GPR54 receptor expressed in GnRH neurons3, causing the pulsatile secretion of GnRH into the HPG circulation. This stimulates the synthesis and release of LH and FSH in the adenohypophysis, which will eventually reach the gonads. FSH initiates spermatogenesis in men, while LH stimulates testosterone production by Leydig cells4. This axis is regulated by a negative feedback system mediated by estrogens4. Recent research suggests kisspeptin may be the mediator, as estrogens decrease its expression5.
Although estradiol has been isolated and identified as a sex hormone since 19236, its role in male sexual function has only recently been understood. Aromatase knockout mice (Cyp19KO or ArKO) were studied to assess their fertility, revealing alterations in copulatory behavior consistent with prior research, and highlighting the importance of local estrogen synthesis in the medial preoptic area during development. When placed with a female mouse, these ArKO mice exhibited a curious and insecure behavior, initiating what appeared to be a non-sexual relationship with females. Fertility deterioration was also observed by the number of litters; wild-type mice had an average of 1.75 ± 0.3 L. whereas of the ArKO mice, 5 had an average of 1.2 ± 0.2 L and the rest produced no offspring. These differences were attributed to alterations in spermato-genesis caused by estrogen deficiency, potentially damaging sperm fertilization potential and reducing copulatory behavior7.
Male erectile function relies on a complex interplay between various factors including the nervous system, vascular endothelium, hormonal, psychological factors, and structural integrity of the penis. Sexual stimulation activates the parasympathetic system leading to the production of nitric oxide (NO), causing cavernous sinuses and arteries to dilate and enhancing blood flow. Prostanoids and hyperpolarizing factors from penile endothelium allow the filling of the sinusoidal spaces of the corpus cavernosum. Compression of the venules of the sub-tunica generates complete occlusion of venous drainage, sustaining the erection8. Hormonal influences include estrogens, which increase NO synthase expression, and testosterone, crucial for penile structural integrity9. However, very high serum concentrations of estrogens are associated with erectile dysfunction (ED), which could be attributed to a decrease in gonadotropins due to negative feedback in the axis10. Testosterone deficiency, on the other hand, can impact NO-mediated muscle relaxation, apoptosis of smooth muscle cells, formation of adipose tissue deposits associated with fibrosis of the cavernous bodies, and damage to the integrity and functionality of the endothelium11, all of which would ultimately alter erectile function.
Likewise, libido can be influenced by psychological, physical, and endocrinological factors. The medial preoptic area of the hypothalamus is central to its processing, with testosterone playing a vital role10. In severely hypogonadal men, a 6-month exogenous testosterone treatment increased libido and nocturnal erections12. While testosterone treatment in eugonadal men raised self-reported libido despite no changes in the frequency of sexual activities, hypogonadal patients experienced a significant increase in sexual activities13. Furthermore, exogenous estradiol impacts male libido differently based on testosterone levels. When testosterone is low, exogenous administration of estradiol can increase libido14. A study involving 202 male subjects showed that suppression of endogenous androgen production led to decreased libido and erectile function, revealing that estradiol concentrations greater than 10 pg/mL help maintain libido, indicating a preference for aromatizable androgens in hypogonadal men15. However, the effect of estradiol supplementation on sexual function in eugonadal men remains inconclusive14.
This study aims to determine the association between the estradiol-to-testosterone (E/T) ratio and male sexual dysfunction (SD), to highlight its importance and the potential consequences of its alteration on male sexual function.
Materials and methods
This is a case-control study, defining cases as adults with hypoactive sexual desire, with or without SD. Sample size determination: 1:1 ratio, with a total of 32 patients. Exposure parameters: frequency of exposure among cases 0.14; frequency of exposure among controls 0.60; odds ratio to detect 2.00; confidence level 0.95; power 0.80. Subjects were male patients over 18 years old with hypoactive sexual desire or ED with chronic non-communicable diseases (NCD). Patients undergoing hormonal treatment, users of anabolic steroids or androgen receptor modulators, with chronic liver pathology, or using psychotropic drugs were excluded.
Data are presented with descriptive and inferential analysis, mean differences were calculated and associations between variables determined. Establishing the odds ratio (OR) for low or normal T values (reference value 3.46 ng/mL16). Testosterone values were adjusted for age. LH was classified as normal or inappropriately normal. A subanalysis was performed according to the presence of hypogonadotropic hypogonadism (HH). Mean differences between groups were analyzed for E/T ratio, testosterone, and estradiol. A correlation analysis was generated between estradiol, testosterone, and E/T ratio values and BMI. A correlation between E/T ratio and sexual steroid values were included in the study. Finally, the usefulness of E/T ratio measurement in the evaluation of SD (ED and hypoactive sexual desire) and HH was analyzed using ROC curves and likelihood ratio (LR) analysis. Statistical significance was considered at p < 0.05. IBM SPSS Software 2019 was used for this analysis.
Data collection techniques
Hormonal determinations were performed after blood extraction. The electrochemiluminescence method was used for estradiol, FSH, LH, total testosterone, and prolactin. The latter was obtained from a pool of three samples, obtained at intervals of 20 min. The IIEF (International Index of Erectile Function) (17 questionnaire was used to assess the condition of ED.
Results
Table 1 presents the descriptive data of the subjects. There is a difference in age distribution between the groups, with mean age being higher in the cases group. The lower IIEF score is observed in the cases group, which was a selection criterion.
Table 1 Subjects descriptive data
| Controls | Cases | |||
|---|---|---|---|---|
| Mean ± SD | Range | Mean ± SD | Range | |
| Age | 38 ± 16 | 19-77 | 52 ± 12 | 30-73 |
| T | 4,97 ± 3,14 | 2,15-13,56 | 3,87 ± 3,55 | 1,01-12,37 |
| corrected T | 5,56 ± 3,22 | 2,16-13,98 | 5,05 ± 4,74 | 1,48-16,56 |
| E/T Ratio | 5,41 ± 3,51 | 0,50-14,8 | 8,04 ± 6,44 | 1,40-27,70 |
| E2 | 20,28 ± 8,51 | 5,27-34,3 | 18,85 ± 6,96 | 7,30-30,96 |
| FSH | 5,38 ± 4,13 | 1,52-13,5 | 4,32 ± 2,65 | 1,42-10,8 |
| LH | 4,71 ± 2,88 | 1,99-11,78 | 3,30 ± 1,46 | 1,02-5,53 |
| IIEF ED | 19 ± 2 | 17-24 | 12 ± 6 | 4-21 |
| IIEF Libido | 8 ± 1 | 7-10 | 5 ± 3 | 2-8 |
| BMI | 29,26 ± 6,24 | 20,37-41,80 | 29,01 ± 4,66 | 22,86-42,39 |
SD: standard deviation; IIEF ED: IIEF erectile dysfunction dimension; IIEF libido: IIEF sexual desire dimension; T: testosterone; E2: estradiol; FSH: follicle stimulating hormone; LH: luteinizing hormone; BMI: body mass index.
For the normality tests, a significance value of the test above 0.05 rejects the hypothesis of a non-normal distribution of the variable and accepts the null hypothesis of a normal distribution. The Shapiro-Wilk test was used for small sample sizes. The hypothesis of a normal distribution was rejected for the variables estradiol/ testosterone ratio (E/T ratio), testosterone, and LH with a p < 0.005, therefore the difference between cases and controls was evaluated using non-parametric Mann U tests. The F-test variances were similar for both variables between the cases versus control subgroups (p > 0.05).
OR and LR were established for testosterone and E/T ratio variables in relation to the cases and control subgroups. The relationship between the subjects' baseline conditions with the presence or absence of alteration in IIEF in the ED and libido dimensions was established (Table 2). No association was found between smoking status and HTN with ED or changes in libido. A significant LR of 6.58 was observed for DM.
Table 2 Base conditions OR and LR
| OR | CI 95% | p | LR | p | |
|---|---|---|---|---|---|
| Smoker | 0,467 | 0,04-5,73 | 0,256 | 0,37 | 0,541 |
| HBP | 4,2 | 0,70-25,26 | 0,418 | 2,76 | 0,96 |
| DM | 11,67 | 1,23-110 | 0,125 | 6,58 | 0,037 |
HPB: high blood pressure, DM: diabetes mellitus.
The non-parametric test analysis revealed a significant difference in testosterone levels between cases and controls. There is no significant difference between estradiol or E/T ratio (Table 3).
Table 3 Evaluation of the differences in mean
| Controls | Cases | p | |||
|---|---|---|---|---|---|
| Mean ± SD | IC 95% | Mean ± SD | CI 95% | ||
| T | 4,97 ± 3,14 | 3,29-6,64 | 3,87 ± 3,55 | 1,97-5,76 | *0.02 |
| Tc | 5,56 ± 3,22 | 3,38-7,27 | 5,05 ± 4,74 | 2,52-7,57 | 0,09 |
| Estradiol | 20,28 ± 8,51 | 15,74-24,82 | 18,85 ± 6,96 | 15,14-22,55 | 0,64 |
| Ratio E/T | 5,42 ± 3,51 | 3,53-7,28 | 8,04 ± 6,44 | 4,61-11,47 | 0,20 |
T: testosterone; Tc: testosterone corrected for age; E/T Ratio: estradiol/testosterone Ratio; SD: standard deviation.
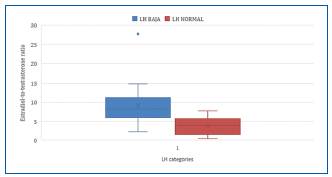
Subgroups of low and normal testosterone and normal and inappropriately normal LH were established (Fig. 1). A significant OR of 13 was found for low testosterone and inappropriately normal LH variables, and a high and significant LR was found for both variables. An OR of 5 was found for testosterone adjusted for age.
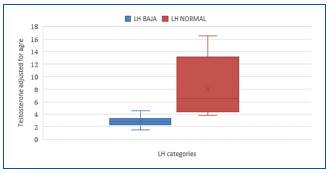
The categories of normal LH and inappropriately normal LH were analyzed, observing a significant difference E/T ratio and testosterone adjusted for age (Table 4).
Table 4 Hormonal levels comparison according to LH response
| LHin | Normal LH | |
|---|---|---|
| Mean | Mean | |
| E/T Ratio | 9,72 | 4,69 |
| Tc (ng/dL) | 2.09 | 6,01 |
LH: luteinizing hormone; E/T Ratio: estradiol/testosterone ratio; Tc: testosterone corrected for age.
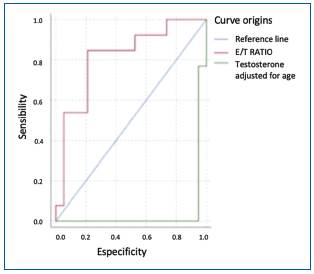
ROC curves were generated to analyze the effectiveness of E/T measurement in the diagnosis of HH, and as a parameter for evaluating SD (Fig. 2). A cutoff point of 5.98 for the E/T ratio was used, which approximates to 6, with a sensitivity of 0.85 and a specificity of 0.79.
Discussion
Table 1 shows a higher average age in the case group, consistent with the established link between age and SD prevalence18. The origin of organic ED can be classified into two groups: endocrine and non-endocrine, with the latter including NCDs, mainly cardiovascular risk factors. In our population, although there is a higher prevalence of DM and HTN in the case group, it is not statistically significant in the context of NCDs in the population. These findings partially resemble those of Dunajska et al., who observed low hormone levels and metabolic syndrome in men with arteriosclerosis19. Our study did not find significant differences in hormone values, likely due to similar NCD prevalence between the groups.
LR for smoking in this population does not show an association with SD possibly due to the sample size. Larger studies are suggested for confirmation. DM is a significant risk factor for SD with a statistically significant LR value over 6 (Table 5), in line with Carrillo's study that found an OR of 2.71 (95% CI 1.57-4.66) and suggested ED diagnosis preceded DM diagnosis20.
Table 5 Association between testosterone levels and sexual dysfunction
| Cases | Controls | OR | CI 95% | |
|---|---|---|---|---|
| Low T | 81.3% | 25% | 13 | 2.39-70.4 |
| Low corrected T | 62.5% | 25% | 5 | 1.1 - 22.8 |
| LH in | 81.3% | 25% | 13 | 2.39-70.4 |
T: testosterone; Tc: testosterone corrected for age; LH in: luteinizing hormone inappropriately normal.
HTN shows no significant association with cases. However, there is a clear association between HTN and HH, with an LR of 19, possibly due to changes in gonadal vascularity causing hypoperfusion, and further evaluation through Doppler ultrasound is suggested.
Significant associations between SD and testosterone were observed, with an OR of 13 (2.39-70.4) p = 0.003. The positive association remains after adjusting for age with an OR of 5 (1.1-22.8) p = 0.04 (Table 5). However, only adjusted testosterone levels show statistical significance in the mean difference analysis (Table 3), while E/T and E2 do not.
Castello conducted a prospective study of 230 patients to evaluate the gonadal profile and IIEF questionnaire for libido. The mean age was 66.32 ± 8.17 years, with 46.5% experiencing decreased libido. They observed a positive linear association between age and free and bioavailable testosterone with ED and increased libido. Age was the only independent variable for both ED and libido. No association was found between E/T imbalance and SD21, which agrees with the present study and validates the evaluation of testosterone in SD. Similarly, Wu found a higher E/T ratio in subjects with SD compared to the control group in a case-control study of 878 patients in China, with a significantly higher proportion of ED compared to premature ejaculation22.
When reviewing the result of the sub-analysis of the relationship between sexual steroid levels and the normal or inappropriately normal LH response, a significant and strong difference is found between the average values of the ET ratio (9.72 ± 6.53 vs. 4.68 ± 3.34 p = 0.002) and T adjusted for age (2.62 ± 0.61 vs. 7.14 ± 4.3 p < 0.001), according to the LH response (Table 6), with no significant difference observed in E2. The results suggest that measuring the E/T ratio is useful for studying the inappropriate LH response with an LR of 13.51, p < 0.001 (Table 7).
Table 6 Differences in mean according to LH response
| LHin | Normal LH | |||||
|---|---|---|---|---|---|---|
| Mean | CI 95% | Mean | CI 95% | |||
| Corrected T | 2.62 ± 0.61 | 2.25-2.98 | 7.14 ± 4.3 | 5.07-9.21 | < 0.001 | |
| E2 | 18.43 ± 7.65 | 13.8-23.05 | 20.34 ± 7.81 | 16.57-24.1 | 0.5 | |
| Ratio E/T | 9.72 ± 6.53 | 5.94 ± 13.48 | 4.68 ± 3.34 | 3.07-6.29 | 0.002 | |
E2: estradiol; E/T Ratio: estradiol/testosterone ratio; LHin: inappropriately normal luteinizing hormone; LH: luteinizing hormone.
Table 7 E/T Ratio OR and LR according to LH response
| LHin | Normal LH | OR | CI 95% | p | LR | p | |
|---|---|---|---|---|---|---|---|
| High E/T ratio | 84.60% | 21.10% | 20.62 | 3.18-133.44 | 0.014 | 13.51 | < 0.001 |
E/T Ratio: estradiol/testosterone ratio; LHin: inappropriately normal luteinizing hormone; LH: luteinizing hormone.
Regarding ED severity, a study on 52 patients examined the association between E/T ratio and ED severity, finding that an increase in E/T ratio was linked to a decrease in erection duration. After adjusting for confounding variables, they found that every unit increase in E/T ratio reduced the duration of penile root erection by 4.34 min23. In a study with a similar aim, El-Sakka found a significant association between ED severity and hormone levels. Patients with low T levels and elevated E2 levels had lower IIEF scores in the erectile function dimension24. Although this association was not evaluated in the present study, it arises as a recommendation for future research.
Studies have found associations between the E/T ratio and anthropometric parameters. In a 2013 study of 821 men aged 30-79 years, longitudinal analysis did not show significant associations between baseline hormone levels and changes in anthropometric measurements over time. The results imply that adiposity has a greater impact on hormone levels than the contrary, as no correlation was observed between baseline hormone levels and changes in BMI or other measurements during the 4.8-year follow-up period25. Fejes investigated the effect of BMI on hormone and semen profiles of subfertile men with oligospermia. Sperm concentration and reproductive hormone levels were compared between groups divided by BMI. The E/T ratio was significantly reduced in the high BMI group when compared to the normal BMI (17.4 vs. 12.3; p < 0.05), as was sperm concentration26. Similarly, Wu et al. found an inverse association between the E/T ratio and change in fat mass during a 5-year follow-up of Australian adults27, while Baydilli et al. reported a negative correlation between total testosterone and E/T ratio with BMI28. Our study found no significant linear correlation between BMI and hormonal variables.
Iacono studied 47 patients with ED using the IIEF score and Rigiscan® evaluating total testosterone, free testosterone, and cavernous body biopsy: a significant difference was found in age, collagen fiber percentage, and testosterone levels between patients with positive Rigiscan (PR) and negative Rigiscan (NR). Hypogonadism increased the risk of ED with OR: 15.5, 95% CI: 13.4-17.6. in elderly patients29. The present study shows a similar association with OR: 13 (2.39-70.4) p: 0.003 for T and OR: 5 (1.1 -22.8) p: 0.04 for T adjusted for age. Iacono also analyzes the usefulness of testosterone in the evaluation of ED with an ROC curve showing an area under the curve (AUC) of 0.9129. In the present study, the ROC curve (Fig. 3), with an AUC of 0.82, favors the evaluation with the E/T ratio in HH and 0.63 (Fig. 2), for the study of the E/T ratio in ED although similar results have not been found in the literature.
Conclusion
A statistically significant difference was found in testosterone values in SD, but beyond that, the utility of the test is established by a significant LR, increasing pre-test probability. The E/T ratio becomes relevant with a favorable ROC curve and significant LR in late-onset HH. The ROC curve serves as a performance indicator for the test, and the LR acts as a supportive factor for pre-test probability analysis and positive predictive value. Measuring estradiol in the initial approach would not be advisable, it should be done if testosterone falls below the reference value of 3.5 ng/mL adjusted for age. Studies evaluating the E/T ratio across different age groups are needed to establish cutoff points. Controlled clinical trials for patients with HH secondary to elevated E/T ratio are justified as well as evaluating possible pharmacological interventions.