INTRODUCTION
Pericarditis is rarely seen in pediatrics, and few reports on this condition in infants are found. 1 Mortality rates of bacterial pericarditis are high, which may be associated with secondary dissemination of other infectious foci such as pneumonia, meningitis, osteomyelitis, septicemia or subdiaphragmatic abscesses. 2-4
In general, the symptoms and signs of bacterial pericarditis are non-specific, thus making timely diagnosis difficult, which is associated with poor prognosis. The diagnostic suspicion, in this case, led to early diagnosis and favorable clinical outcome.
CASE PRESENTATION
A 10-month old male patient from Bogotá-Colombia (urban area), weighing 8 kg and mestizo was admitted to the emergency department with fever of two days (38.5°C) and respiratory distress. No history of importance and complete vaccination for his age were reported. The child was hospitalized in another institution two months before hospital admission due to bacterial pneumonia managed with crystalline penicillin. Since then, he was an ambulatory oxygen user.
On admission, the patient presented: heart rate of 186 beats per minute; no hypotension (blood pressure at 106/65 mmHg); respiratory frequency of 50 breaths per minute; fever (38.3°C); SaO2 in 74% with oxygen at one liter per minute; mucocutaneous pallor; somnolence; no jugular vein engorgement; audible, rhythmic and tachycardic heart sounds; no pericardial rub, rhonchi and crepitus in both lungs; subcostal and suprasternal retractions; no hepatomegaly or edema; peripheral pulses and capillary refill without alterations, which required increased oxygen delivery (FIO2 0.5). His hemogram showed leukocytosis of 30 230mm3 with polymorphonuclear leukocytes of 24 360mm3, thrombocytosis (platelets of 1 006 000mm3); 5.8g/d serum proteins, and increase in acute phase reactants: C reactive protein of 96mg/L and procalcitonin of 5.24ng/ mL. Initial chest X-ray showed cardiomegaly, bibasilar and retrocardiac parenchymal opacities, without signs of pulmonary congestion or pleural effusion (Figure 1).
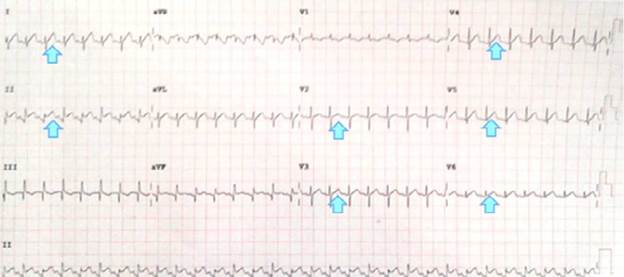
Pericardial effusion, myocarditis and dilated cardiomyopathy were suspected considering septic status and cardiomegaly, since they are differential diagnoses associated with multilobar pneumonia. However, no signs of pulmonary hyperflow, cardiac failure or tamponade or pulmonary or systemic congestion were observed which, added to electrocardiographic changes (Figure 2) that showed repolarization and supra-ST elevation disorder in V2 to V6, DI and DII (evident changes in the early stages of pericarditis), led to suspect pericarditis associated with pericardial effusion, without cardiac tamponade, in the first place.
Source: Own elaboration based on the data obtained in the study.
Figure 2 Evidence of electrocardiographic changes in the patient.
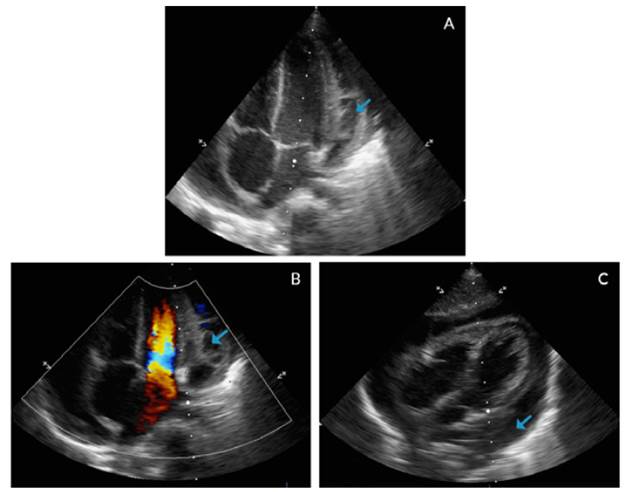
In addition, an echocardiogram showed global pericardial effusion and the presence of thick membranes between the visceral and parietal pericardium, without signs of cardiac tamponade, adequate ventricular function, ventricular ejection fraction of 77% and central venous pressure of 8-10mmHg, without signs of pulmonary hypertension (Figure 3).
Source: Own elaboration based on the data obtained in the study.
Figure 3 Echocardiogram with apical four chamber view (A, B) and coronal axial plane (C).
Respiratory distress, fever, tachycardia and altered state of consciousness without hypotension or alteration in distal perfusion were interpreted as a septic shock of pulmonary origin in hyperdynamic phase. The septic pattern and the echocardiographic findings increased the diagnostic probability of purulent pericarditis, so management with ceftriaxone (400 mg IV every 12 hours) and vancomycin (360 mg IV a day) was initiated. Then, the pediatric surgery service performed a pericardiotomy in the operating room and obtained about 50mL of turbid fluid with whitish membranes. Pericardial fluid analysis showed 2 600mm3 of polymorphonuclear leukocytes at 90%, monocytes at 1 0%, glucose of 0.2 mg/dL, lactate dehydrogenase (LDH) of 591 IU/L, fresh red blood cells of 1 500, crenated red blood cells at 2 080 and total protein of 5.23 g/dL (pericardial fluid/ serum proteins ratio: 0.9), which suggested a bacterial etiology.
Following the surgical procedure, a right jugular central venous catheter was passed (central venous pressure was not measured) and coupled to mechanical ventilation (CPAP PS), without requiring vasoactive support. The patient was admitted to the pediatric intensive care unit in normotensive condition, with tachycardia, adequate distal perfusion and under the effects of sedation and anesthesia (midazolam and fentanyl). Transfusion of 120cm3 of red blood cells was carried out when arterial blood gas analysis yielded a figure of 7.1gr/dL.
Extubation was achieved within a few hours with adequate response, while fever was observed until the day after antibiotic management was initiated. The control blood count at that moment showed decreased white blood cell count, polymorphonuclear cells and platelets in relation to the previous exam (leukocytes: 24 600mm3, polymorphonuclear leukocytes: 13 530mm3, Hb: 11.4 g/dL, HCTO: 35%, platelets: 820 000mm3) as well as decreased procalcitonin levels (5.21 ng/mL). The pericardiostomy tube was maintained for 4 days and blood cultures, as well as Ziehl-Neelsen, KOH and pericardial fluid culture tests, were negative.
To reduce the risk of toxicity and before clinical improvement, management with vancomycin was adjusted to clindamycin (80 mg IV every 6 hours) to complete 4 weeks of treatment.
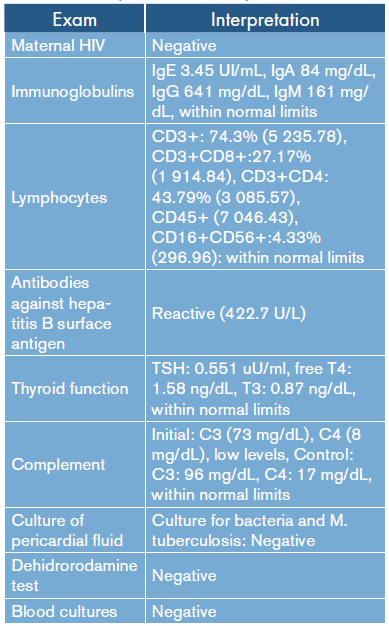
Studies to rule out immunological involvement were within normal limits (Table 1). Given the cyclic and echocardiographic improvement, discharge was authorized (Figure 4).
Table 1 Interpretation of laboratory results.
Source: Own elaboration based on the data obtained in the study.
DISCUSSION
Purulent pericarditis is rarely observed in pediatrics and its incidence has not been properly established in this population. Most studies are documented in case reports and reports in infants are scarce. 5-10
A study conducted in Uruguay 1 followed 19 pediatric patients with pericarditis, finding that 9 of them had a purulent origin and only 2 were younger than 12 months. This research showed that bacterial etiology is <50% and its occurrence in infants is unusual.
Considering a history of previous infections such as pneumonia, meningitis, osteomyelitis or subdiaphragmatic abscesses is important in children with purulent pericarditis. 2 In this case the infant presented bacterial pneumonia, which required hospitalization 2 months prior to this event.
Upon admission, the patient presented with respiratory distress, fever, tachycardia and increased oxygen requirements, which was explained by a septic pattern of pulmonary origin and pericarditis. Chest pain, despite being one of the most common symptoms in adults and older children, is difficult to interpret in infants. Clinically, there were no signs of cardiac tamponade (hypotension, jugular vein or veiled cardiac sounds), a complication that increases mortality and is explained by volume, accumulation rate, and the nature and etiology of the exudate between the pericardial layers that compress the cardiac chambers. 11
The initial chest X-ray showed bibasilar and retrocardiac consolidations. The cardio-thoracic index was 0.66 (>0.55) and was interpreted as cardiomegaly considering the patient's age. There was no evidence of pulmonary hyperflow or pulmonary venous congestion. The latter, together with electrocardiographic alterations (repolarization disorders compatible with acute pericarditis) and absence of signs of heart failure (no signs of systemic or pulmonary congestion or low cardiac output), led to rule out pericarditis and associated pericardial effusion, without cardiac tamponade at first. 3,12
The echocardiogram is the diagnostic method of choice to clarify radiological findings. 2,3 In this case, it showed a global pericardial effusion associated with the presence of thick membranes between the visceral and parietal pericardium, without cardiac tamponade, systolic-diastolic function involvement or alterations in the myocardium. Computed tomography (CT) is indicated when emergency echocardiogram is not available and when inconclusive echocardiographic results, poor response to treatment, atypical presentation, penetrating lesion, suspicion of neoplasms and pulmonary infections or mediastinitis are observed. 13 This patient did not require chest CT because of his rapid improvement, which reduced the possibility of complications.
Cytological and biochemical evaluation of pericardial effusion can help to obtain a diagnosis. The increase of LDH, the protein ratio in the pericardial fluid and serum >0.5 are characteristics that can lead to conclude that the fluid is compatible with the exudate. 14
The protein ratio of the pericardial fluid and the serum proteins was 0.9 (>0.5), which suggested exudate characteristics. In the culture of this liquid, no bacterial isolation was obtained, despite the presence of purulent pericardial fluid. Some possible explanations include that these cultures are based on the detection of bacteria in the planktonic form (individual bacterial cells), but, over time, it has been demonstrated and accepted that the bacteria responsible for infections (including Staphylococcus aureus and Streptococcus pneumoniae) are associated in the form of biofilms, in such a way that they guarantee their survival by changing their properties and enhancing their virulence factors.
Associated bacteria in biofilms yield a negative result in culture tests directed at individual bacteria. 15 For this reason, it is necessary to use different diagnostic tests that can provide a fast identification of microorganisms, including PCR (polymerase chain reaction) 16 and ELISPOT (enzyme-linked immunospot) 17; the latter detects T cells specific for microbacteria and other microorganisms.
The treatment of purulent pericarditis includes pericardiocentesis, which is indicated for diagnosis and treatment, especially in the presence of significant effusion. 2-4 In this case, the septic status and the presence of pericardial effusion together with adherent membranes suggested purulent pericarditis. Empirical antibiotic treatment initiates with vancomycin and ceftriaxone and is maintained for 4 to 6 weeks, in order to eliminate the main microorganisms involved, namely, S. aureus, Haemophilus influenzae and S. pneumoniae.2,3 In addition, bed rest and management with NSAIDs are recommended 2-4. In this case, antibiotic management with vancomycin was adjusted to clindamycin to reduce the risk of toxicity and support clinical and imaging improvement.
Other condition that can be considered for a pediatric patient presenting with this pathology is immunodeficiency, either primary or acquired and sometimes associated with malnutrition. 18 The main immunological etiologies were discarded for this patient and no degree of malnutrition was documented during the assessment.
The limitations of this study include the lack of availability of the clinical records of the patient prior to admission and non-ambulatory follow-up of the patient. On the other hand, its strengths include comprehensive and interdisciplinary management, as well as permanent imaging support throughout the recovery process.
CONCLUSIONS
The relevance of this case lies in the presentation of the disease at a rare age. Signs and symptoms are non-specific, so high clinical suspicion is required based on the symptomatology and clinical and paraclinical findings that include radiography, electrocardiogram and echocardiography. 2-4 Timely diagnosis and early treatment decrease the probability of complications and favor better results.
















