1. Introduction
The excessive demand and single-use of plastic worldwide has led to a high accumulation of plastic waste in water and soil. According to [1], about 8 million tons of plastic end up in the ocean each year. The negative impacts of plastic pollution for ecosystems and public health have been highly documented due to the excessive ingestion of both plastic and microplastics by humans and animals. There is an ever more serious concern about toxic substances in the plastic itself or its additives that act as carcinogenic compounds or endocrine disruptors.
Yet, the use of plastic persists as it has clear advantages over other materials such as glass, paperboard, or wood. In situations including the COVID-19 pandemic, plastic has proved to be especially helpful thanks to its aseptic conditions, resulting in a higher use and, consequently, increasing the Plastic Waste Footprint (PWF) worldwide [2]. Despite that, the fact this material does not degrade for more than 700 years is a greater disadvantage as this is a difficult situation to manage: “only 9% of plastic used in the world is recycled” [3]: 42.4% in the E.U., 8.4% in the U.S. and 10% in South America [4-6].
Therefore, research on easily biodegradable polymeric substitutes has increased since petrochemical plastics must be replaced to decrease the negative impacts of waste. The consumer demand and production of bio-based plastics is expected to increase 24% from 2.11 million tons in 2018 to 2.62 million tons in 2023 [7]. Starch-based thermoplastics constitute the largest market, followed by poly (lactic acid), PLA, and the polyhydroxyalkanoates (PHA) [8]. This study focuses on the bioplastic synthetized of xyloglucan of tamarind kernel powder (XgT) in a 50:50 ratio with ethyl acrylate (EA), obtained [9] during the research of substitutes for polystyrene.
The purpose of this document is to determine whether b-XgT is biodegradable and, thus, whether it is a good substitute for polyethylene. Considering biodegradation of plastics is usually measured by the conversion of organic carbon into CO2 under controlled laboratory conditions, a standard test has been developed for this purpose, since under real soil and composting conditions it is not possible to measure CO2 release [10]. Additionally, because composting has been reported as a viable means of disposal for biodegradable plastics [11], the guidelines from the standard method 5338 [12] were implemented to measure the biodegradability of b-XgT in compost. However, the method was modified to anaerobic conditions, and different quantities were set due to the limited quantity of the sample.
The compost was obtained from the composting plant in the National Autonomous University of Mexico (UNAM, for its acronym in Spanish), which dedicates itself to the biological treatment of solid urban waste from the UNAM university city. This consists of vegetable waste from pruning, grasses, weeding plants, logging for sanitation, restaurant waste, food modules, and the consumption of students and teachers. Because the plant has been in operation since 1994, the standardized process secures microbial richness made up of millions of specimens [13].
While there are diverse methods to determine the biodegradability of plastics from renewable resources [14], the microorganisms must generally excrete extracellular enzymes to depolymerize the available nutrient of that nature and, therefore, transport it to their cellular mass. The result is that H2O, CO2, CH4 (anaerobic conditions), inorganic trace compounds, and biomass [15] are obtained. The present study focused on measurements in the laboratory, prioritizing this over other measurements including field and simulation. The criteria taken into account were: loss of mass, CO2 release and registers of visible bioreactor changes.
To evaluate its biodegradability, the b-XgT was subjected to several tests to verify if it can replace the petrochemical polystyrene in wide applications including packaging, energy technology (electrical insulator), communications technology (in casing parts), automobile or toys parts, refrigeration equipment, gardening pots and as copolymer.
2. Materials and methods
The study was conducted using the following procedures according to ASTM 5338 (modifications and specifications are mentioned):
2.1 Determination of initial parameters
Prior to mounting the bioreactors in order to identify whether compost and sample (b-XgT) present adequate conditions or set them, initially, the compost was homogenized with the manual removal of big particles and the sieving through orifices 5x5 mm2 wide. The authors found that with the 3 and 10mm wide size, friable texture and moisture retention are assured.
The following were determined for the compost and experimental sample: the total solids (APHA 2540D) and the volatile solids (APHA 2540E; [17]) with Thermolyne Type 1500 Muffle Furnace. The compost’s pH (ASTM D3987) with an Oakton pH110 multiparameter, in 3 consecutive readings was also determined. The quantity of C, H, N, and S for the experimental sample was determined through the elemental analyzer PerkinElmer 24000, using the technical procedure PTUSAII-FQ-AE-001 from the Unit of Support Services for Research and Industry (USAII, for its acronym in Spanish), from UNAM.
2.2 Description of the problem substances’ behavior
The changes of test substances were traced through photographic records and descriptions for each bioreactor, mainly the one with b-XgT. The one with positive (cellulose) and negative (polyethylene) controls were for support and verification.
2.3 CO2 sample measurement
2.3.1 Method
The composting systems (bioreactors) were conformed in flasks with the test substances, in a 1:9 ratio with compost, as follows:
Blank control: 45g of dry weight compost.
Positive control: 45g of dry weight compost + 5g of dry weight cellulose (cut from laboratory craft paper in 5x5 mm2).
Negative control: 45g of dry weight compost + 5g dry weight polyethylene, cut in 5x5 mm2.
Duplicated sample (M1 y M2): 45g of dry weight compost + 5g of dry weight of XgT bioplastic in 50:50 ratio with EA.
CO2 calculation was made from sample measurements by means of a bubble system directed to the Ba(OH)2, 0,024N (gas trap) for 30 minutes, every two days, as indicated in Fig. 1. In the remaining time, the flasks were placed in a Labnet 211DS incubator at 58°C±1°C. The complete test was carried out and then duplicated.
Based in ASTM 5338, reactions occur at the moment CO2 enters the flasks with Ba(OH)2 and the BaCO3 produced is titrated with HCl and phenolphthalein indicator. .The quantity of CO2 in mmoles was calculated using Eq. (1):
Where: QtyCO
2
= is the quantity of CO2 expressed in mmoles. Vtit(Ba(OH)2 ) = titrant volume used in “CO2 gas trap”, each occasion, in mL. VtitSustProb= titrant volume, in mL. [HCl]= titrant concentration, normality (N) of 0.05

 = molar mass of CO2, in g.
= molar mass of CO2, in g.
2.2 Statistical analysis
Descriptive statistics, normality tests, parametric analysis of variance (ANOVA, mainly of repeated measures), and multiple comparisons by post hoc analysis were performed. Accordingly, non-parametric tests such as sum of Wilcoxon, Kruskal-Wallis, Friedman and Spearman correlation coefficient were made.
2.2 Final stage of the test
It was ascertained whether the degradation process in composting had been suitable by establishing if the result was pH>6. Also, loss of mass was determined in relation to the beginning and end of the test, as in Eq. (2).
Where: %WL= Weight loss in %. M 0 = Initial dry weight from the system in g. M f = Dry weight from the system at the end of the test, in g.
3. Results and discussion
3.1 Results from the initial parameters
3.1.1 Preparation of the compost inoculum
A friable structure was obtained by homogenizing the compost from the UNAM compost plant. This characteristic was favorable for gas exchange, microbial activity, pH neutralization, temperature regulation, leveling of water evaporation, and generation of electrostatic attraction for nutrient exchange [18].
3.1.2 Determination of the pH
The values for pH of the compost inoculum were located in the optimal range of 7.0 to 8.2 [12], as shown in Table 1.
Considering pH could be a limiting factor for the bacteria metabolism [19], the obtained pH (close to the optimal pH of 7.8) was related to good microbial development.
3.1.3 Elementary content: C, H, N, and S
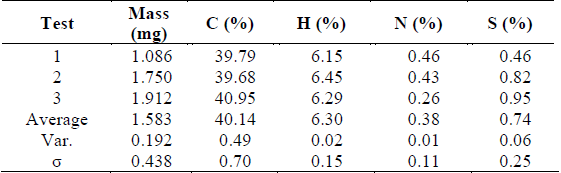
This outcome was duplicated for b-XgT and triplicated for cellulose, given the regularity in the b-XgT data, as shown in Table 2:
The experimental sample of b-XgT
3.1.4 Determination of Total solids (TS%)
From the measurement of total solids, TS=67.85% was obtained for the compost inoculum, which represents a moisture content of 32.15%, hence it was necessary to increase it as the optimum humidity for the highest microbial growth is within a 50-70% range, and the microbial growth decreases under 30% of moisture content. This is due to humidity and is the means of transport for soluble compounds that work as cellular nutrients and as the route for residual products during metabolic activities [21].
For b-XgT, TS=95.48% were obtained, that is, 4.52% of moisture content, which is consistent since it is a solid. There are studies based on thermal, mechanical, and moisture absorption properties in bioplastics, which show that water absorption must be low, between 5 and 7%, to enable injection molding or extrusion with good tensile strength in the material [22].Therefore, the percentage of moisture content obtained in the b-XgT sample is among what is the recommended to obtain good mechanical properties of the new bioplastic.
3.1.5 Determination of Volatile solids (VS%)
Using the residue of the previous procedure of total solids, the volatile solids were determined by calcination at 550°C, VS=20.82% was obtained for compost inoculum and VS=94,58% was obtained in the experimental sample. It is understood that these results are related to the volatilization of organic matter (OM), and in a much lower quantity, to inorganic matter[23], which is useful to appreciate the susceptible matter that biodegrades in composting systems, given the 5% of non-volatile inorganic compounds (Fenton reagent used while b-XgT was synthetized and inorganic remnants). It also considers the chemical composition of b-XgT, a co-polymer of two organic compounds.
3.2 Behavior description of problem substances
The mounted bioreactors, with a 1:9 ratio of substance and compost, showed the behavior described in Table 4.
Table 4 Relevant characteristics during the experimental process
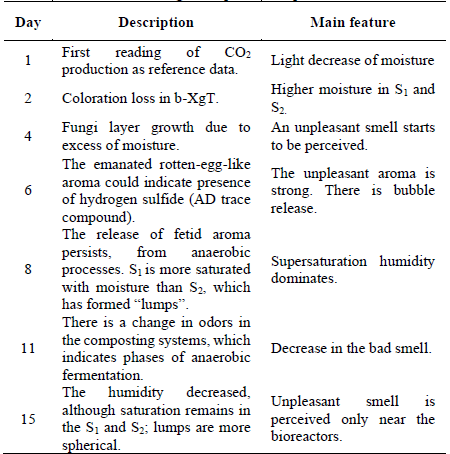
*S1: Sample 1; S2: Sample 2; AD: anaerobic digestion.
Source: Authors.
In order to comprehend the behavior of b-XgT, it must be clear how during controlled degradation in the laboratory there are similar processes to the natural conditions, since as biodegradation advances, micro-environments are created (pH, temperature, nutrients, metabolites and gas). These favor mutualistic processes where growth, reproduction, metabolic processes, and residues from a microbial group, generate a set of suitable nutrients to another [24]. Therefore, if the compost inoculum had by-products such as OM, essential elements and trace elements [25], besides the ones enunciated in Fig. 2, microbial activity began in the bioreactors at the moment of adding the b-XgT.

Source: Modified from [26, 27].
Figure 2 General approach of reactions in the decomposition of waste and aerobic digestion.
During the fermentative process of AD, the metabolic reactions are divided into hydrolysis, fermentation, acetogenesis and methanogenesis. In hydrolysis, microorganisms cooperate to degrade OM in successive stages by means of enzymes that break 1,4-β glycosidic bonds of hemicellulose polymers, such as XgT. They are conditioned by the amount of OM, the presence of volatile fatty acids (VFA), temperature, pH, nutrients, the amount of N and P, among others [24].
Residues (OM+ another nutrients) + substratum (nutr.+ microorg.)+O2+H2O "→" resistant OM+microorg.+ H2 O+heat+ CO2+NO3+trace elem.
Since xyloglucan is a hemicellulose, the structure of which is a linear chain of glucoses (Glc), linked by β(1→4) bonds-lateral residues of xylose and other saccharides-[28]. It is highly susceptible to hydrolysis by microorganisms. Consequently, the fermentation and acetogenesis (acetic acid ions) are useful for methanogenesis [24].
Fig. 3 expresses the products obtained, where CH4, in addition to other trace gases, composes biogas, a renewable fuel comparable to fossil fuels [29].

Source: Modified from [26, 27].
Figure 3 General approach of AD reactions, bioreactors in composting.
The biogas production is considered an additional benefit during the biodegradability test of b-XgT. There is a high potential in large-scale treatment of b-XgT in compost because of the ‘Waste to Energy’ transformation (WTE), since biogas can intervene in cogeneration processes of boiler engines, turbines, electricity generation, or as a biofuel due to its high heat capacity. As part of this process, throughout the anaerobiosis stages, the biogas obtained can be aligned with the global paradigm shift to respond with 30% of the energy requirements by 2023 [30] given the predicted depletion of fossil energies.
3.3 Sample CO2 measurement
The titration volume of HCl during the sample CO2 measurement were processed with Eq. (1) to obtain the quantity of the degradation gas in millimoles, as shown in the next example. The outcomes are displayed in Table 5:
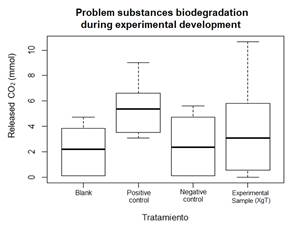
The information above is illustrated in the scatter plot (Fig. 4); time is considered as an explanatory variable and CO2 release as a response variable. A similitude in the curves’ behavior is evidenced until the eighth day, when the positive control degradation curve starts to grow once again due to the persistent degradative processe-even when the other bioreactors indicated a decrease in microbial activity. Nevertheless, there is a negative correlation between degradative activity and time of treatment.
The behavior of the CO2 release curves was similar to the theoretical curves of O2 requirements during the composting process, which indicates the relation between the maximum microbial activity of decomposition of easily degradable compounds [31] or the rupture in the XgT polysaccharide bonds, the availability of which decreased as the 18 days of process elapsed. The degradation of b-XgT is based, on one side, on the nature of xylose, an abundant residue of xyloglucan, that allows adequate anaerobic digestion [32]. Also, compostable polymers can undergo the breaking of glycosidic linkages into controlled systems, where thermophilic organisms supports the high rate of degradation during the first 14 days [14,33].
Biodegradability depends on the specific chemical structure of the polymer, the resulting dynamics such as deterioration from growth of microorganisms on their surface, the monomer fragmentation and the microbial assimilation when the polymer’s compounds are used as energy or a carbon source and on the environment in which they are exposed. For this reason, some polymers are biodegradable but not compostable (the latter are environments with the highest microbial diversities); however, measuring the by-products generated during metabolism, as this paper does, prevents the error of cataloguing a stable compound as degradable [34], which has occurred with lignocellulosic materials that are not biodegradable because of their strong bonds [35].
Instead, some compostable polymers (in laboratory conditions at 58°C and measuring CO2 release) are: poly (lactic acid), PLA, which takes 30 days for a degradation of 40% when the ratio is 70:30 with wood; polyhydroxyalkanoates (PHA), which lose 80% of their mass within 70 days; starch-based ones, which degrade by 73% in 109 days; and polycaprolactone ones, which lose 38% of their mass within six days [8]. In comparison, the b-XgT evaluated in similar environments had a lost 40% of its mass (see ‘final stage of the test’ section) within 18 days of CO2 release. As such, its type of compostable biodegradability is viable, in decomposition terms, in order to become a substitute for polystyrene petrochemical plastic.
3.4 Statistical analyses
The biodegradability test on b-XgT was carried out in duplicate treatment on a bioreactor. In comparison with the other test substances, the positive and negative control, the b-XgT had the highest standard deviation values since, as shown in Fig. 5, the bioplastic presented the minimum and maximum CO2 release during the 18 days of degradation, i.e. from 0 to approximately 11 mmoles per day. For this reason, the data presented a dispersed morphology evidenced by a platykurtic kurtosis.
The ANOVA-RM was selected in order to find the difference between composting bioreactors, as it considers unifactorial intra-subject values and small ‘subject groups’ [36], and shows the dependency between the biological degradation values from composting system of one day in relation to the next due to the intermediate process and different stimulus, such as time. Within a priori tests for ANOVA-RM, the normal distribution is the principal result [37]. To achieve that, considering α=0.05, various methods were used and the results showed a normal distribution. SW was the most relevant since it is “the closest test to the real behavior of data” [38]. The results from Table 6 were obtained through comparing the parametric inferential statistics.
After the experiments, multiple or post hoc comparisons were made, where the adjusted peer analysis of the Bonferroni method, being α '= 0.0083, showed the greatest difference between days 2 and 4 in relation to days 13, 15, and 18, in terms of the degradation of b-XgT. Using the least significant difference (LSD) method, it was shown that the greatest difference existed between the sample and the positive control in relation to the blank and negative control.
Besides, non-parametric inferential tests were performed, and the results in Table 7 were obtained:
3.5 Final stage of the test
At the final stage, the validation by pH and the loss of mass of the tests were determined.
3.5.1 Validation
The pH was determined with the remnants of sample bioreactors (see Table 8).
Because the pH obtained was greater than 7, the test can be catalogued as valid and it is not necessary to make additional measurements such as volatile fatty acids [12]. However, it is necessary for further research to consider that when biodegradable plastics are composted, the degraded polymers can release minor additives. If these do not biodegrade, accumulation can occur, which will then require longer testing to verify whether complete biodegradation occurs and also whether there are long-term risks to the environment.”[39].
In another evaluations, including the biodegradable plastic mulch, when the effects on wheat growth are considered, stronger negative effects were shown when compared to polyethylene [40]; however, that research included bioplastic with a fraction of petrochemical plastic (44.6% of PET). Various studies must be carried out in order to check if there are negative effects on plant growth due to the incomplete degradation of b-XgT.
3.5.2 Loss of mass
Results were obtained through determining the total solid at the end of the test in order to get the dry weight from the total mass. The final weight of 27g was less than the one registered at the beginning of the test, which corresponded, using Eq. (2), to a 40.34% of weight loss-an average of the duplicated sample.
4. Conclusions
Biodegradability in materials is a property given by the arrangement of polymers, their chemical structure, the type of compound, the complexity of their bonds, and the environmental conditions to which they are subjected. In this case, the XgT and EA bioplastic, of organic origin (94.6% of volatile solids), had a degradation time of 18 days, with a 40.34% loss of mass in bioreactors with a 1 to 9 ratio with inoculum of conditioned compost in favorable conditions: friable structure, 7.75 pH, 34.13 C/N and an initial humidity of 50%. The process showed not to generate ecotoxicity but to culminate as valid because a pH of 7.18 was obtained, which is not harmful to health.
This investigation revealed that the b-XgT is an adequate substitute for polystyrene in terms of biodegradability, since it is biodegradable-compostable-under laboratory-controlled conditions, mainly through the anaerobic route, which favors the digestion of components of the copolymer such as xylose. This is recognized for allowing the biodegradation of the components to which it is bonded. Parallelly, biodegradation was determined by the time of CO2 release and the presence of other metabolites, since the available nutrients, microenvironments, and microbial groups vary.
The degrading activity of the microorganisms in the composting system of this study, initially aerobic and then anaerobic, depended on the generation of microenvironments by the species of bacteria whose metabolites favor the environment of others. The b-XgT besides being an adequate substitute for polystyrene in terms of its biodegradability, is also susceptible to energetic valorization through AD.
5. Recommendations
As for ethyl acrylate (EA)-is recommended to be aware of EA carcinogenicity investigations, since according to the US National Library of Medicine and the National Center for Biotechnology Information [41] this compound “is considered harmful if it is exposed continuously for some time.” However, the agencies in charge consider that there is weak or inconsistent evidence on the matter for human beings. It is advisable to avoid direct exposure at the time of copolymer synthesis.
As for the tamarind xyloglucan copolymer with ethyl acrylate-it is also possible to consider the use of starch, which is already widely used for different types of packaging. Additionally, they can be mixed with aliphatic polyesters to improve their processability, biodegradability (facilitates microbial attack), and the elastic modulus. Polyurethane (PU) can participate as a reinforcing agent since starches including cassava increase the breaking effort. Chemical modification methods such as the addition of carbohydrate can be considered for research studies such as those that seek to improve moisture resistance, compatibility, and processability with hydrophobic polymers [42].
This paper highlighted that the modifications made to biopolymers, such as PLA, have granted better properties and better biodegradability [43]. This proposal is confirmed by research studies that have modified XgT polymers ‘regioselectively’ to improve their performance in humid conditions. They found it better than petrochemical plastics, with a controlled change to avoid the use of harmful solvents [44]. The synthesis conditions of the biopolymer, such as the temperature and pressure under which it is made, can improve its physical properties [45].
As for the method-it is suggested to always have a considerable quantity of experimental sample, if possible, greater or equal to 20g, to make the determination of initial parameters easier. This also enables replication of the 1:9 ratio of test substance with the compost inoculum in bigger composting system and, likewise, to conduct other studies such as microbial, traction, resistance, flexion, stress-strain curves, among others, which belong to exactly the same compound evaluated in this paper.
It is recommended that the Ba(OH)2 used comes from a recent acquisition, given that it is a highly sensitive to the environment compound and its deterioration can generate measurement noise. Equally, when conditioning it as stock reagent for tests, one must be extremely careful at the moment of making the 0.024N concentration, since movements can add more CO2 readings than appropriate or that are related to the test.