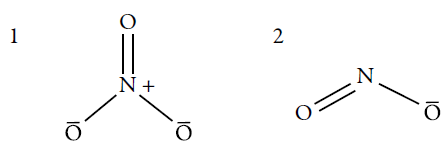INTRODUCTION
Food additives can be defined as substances, such as dyes, antioxidants, thickeners, stabilizers, and preservatives, intentionally added to industrial products in order to provide greater safety and validity to them [1].
Among the innumerable preservatives, nitrates and nitrites are curing salts, used in the preservation of industrial foods, whose intention is to maintain the integrity of the product, that is, to act to delay or inhibit microbial degradation and/or enzymatic degradation of food. These substances also have functionality in terms of color fixing and influencing flavor and aroma, characteristic of cured foods [2].
Nitrates and nitrites are preservatives whose primary activity is the inhibitory action of Clostridium botulinum, a pathogenic bacterium with a high toxic content, capable of inducing foodborne infections in humans, through the production of neurotoxins related to botulism. However, it is important to emphasize that nitrate directly do not have a potential blocker of this bacterium and its reduction to nitrite is essential for this activity [3].
In terms of conservation, these compounds are of great importance for the technological area of food. On the other hand, their intake is controversial as to their harmful effects on human health, especially in relation to the development and progression of cancers [4].
Cancer is the term used to refer to a complex set of distinct and non-communicable diseases, which is characterized by the uncontrolled growth of cells in the most diverse tissues of the body. Once these cancer cells move from one tissue to another, there is the formation of metastasis [5].
It is believed that the carcinogenic potential of nitrates, nitrites, and their derivatives, is related to the ability to damage molecules of deoxyribunucleic acid (DNA) and may contribute to the process of illness with the emergence of diseases such as cancer [6]. However, it is complicated to talk about reducing the levels of preservatives at an industrial level, since the shelf life of food products and their acceptable storage time are also reduced proportionally [7].
Although preservatives have indisputable benefits, the routine use of nitrite and nitrate salts is questionable and worrisome. Thus, the present study aimed to investigate the relationship between the consumption of nitrates and nitrites and the appearance of cancers.
METHODOLOGY
It is a review of literature of the narrative type, in which there was a recovery of articles published in English and Portuguese, in the databases SciELO, Lilacs, Science Direct and Capes journals. Articles related to nitrates and nitrites in foods were included, as well as articles that correlated the consumption of these preservatives with the appearance of cancers, regardless of the year of publication, although articles published between the years 2009 to 2019 were prioritized. In addition, works that could not be accessed in full were immediately excluded from the study.
RESULTS
Nitrates and nitrites are generally associated with industrialized food products; therefore, it is possible to observe the presence of these salts in a wide variety of foods (table 1).
Table 1 Concentration of nitrates and nitrites in different foods.
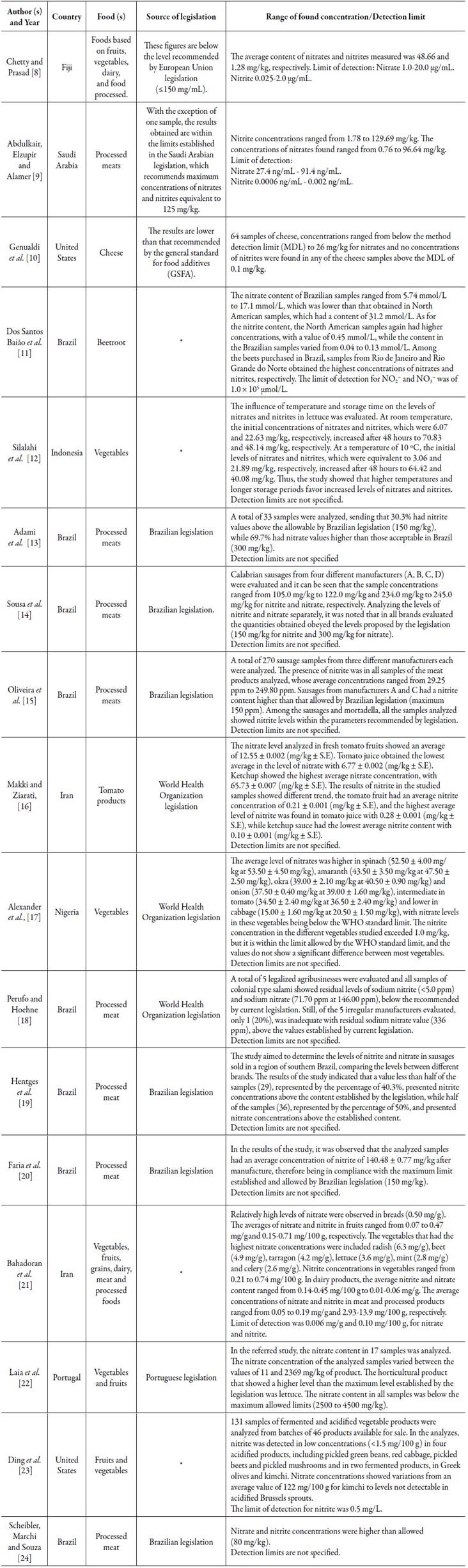
*The study does not cite consulted legislation source; Source: Research data, 2019.
In view of the information described in the table above, it is possible to observe that both meat and processed foods, as well as fruits and vegetables have nitrates and nitrites in their composition, in the most different countries.
It is important to highlight that the concentration of nitrite and nitrate can vary between the samples analyzed, according to the origin (country/region/factory).
In addition, the storage time and temperature itself can contribute to the increase in the content of these preservatives, indicating that the longer the product takes to be consumed, the richer in nitrates and nitrites it can become [12].
Nitrites are mainly associated with meat products, requiring a minimum of 20 mg NaNO2 /kg of product, while nitrates are mostly found in vegetables, corresponding to 70% of the intake of this ion in the human diet, in which is believed to vary between 100 to 200 mg in a conventional diet [2, 25, 26].
Thus, it is important to note that, especially in meat products, the uses of these preservatives are essential to allow the protection of these products against C. botulinum. On the other hand, in fruits and vegetables, their concentrations are influenced by the climate, soil and use of fertilizers. Therefore, there is moderation in the consumption of these foods, especially the industrialized ones that present higher levels of these and other preservatives [2, 12].
Reactions involving nitrates and nitrites after ingestion
The chemical structures of the nitrate and nitrite ions can be seen below (figure 1).
These ions can be found in organic and inorganic form. The organic ones are more complex from the structural point of view, being obtained in a synthetic way, while the inorganic ones are structurally simpler and occur naturally [27].
In the human organism, these compounds appear endogenously (biosynthesis process) and exogenously (water and food) [27, 28]. After ingesting the food or water containing these preservatives, regardless of concentration, there is a natural reaction of reduction by bacterial enzymes (nitrates reductases) and there is a biotransformation of nitrate (NO3) into nitrite (NO2) (equation 1).
Reaction 1 . Biotransformation from nitrate to nitrite by reducing bacteria.
Although nitrate is moderately stable, but nitrite is extremely reactive and this characteristic allows an easy formation of N-nitrous compounds (carcinogens), such as N-nitrosodimethylamine and monomethylnitrosamine, which are about ten times more toxic than nitrates [28-30].
Nitrite is converted into nitrous acid, in the presence of favorable reducing conditions for carrying out the reaction, in which the nitrous acid is reduced to nitric oxide. It is possible to observe the reactions that happen (figure 2), which are potentiated by the acidic pH of the stomach [27, 29].
The consumption of nitrates and nitrites and the appearance of cancers
The excessive consumption of nitrates and nitrites, whether through water or food, has been associated with a great diversity of diseases, stimulating the development of several studies investigating, including, the correlation between the consumption of these preservatives and the appearance of cancers (table 2).
Table 2 Conclusions about the consumption of water and foods rich in nitrates and nitrites and the appearance of cancers, from studies published between 1992 and 2017.
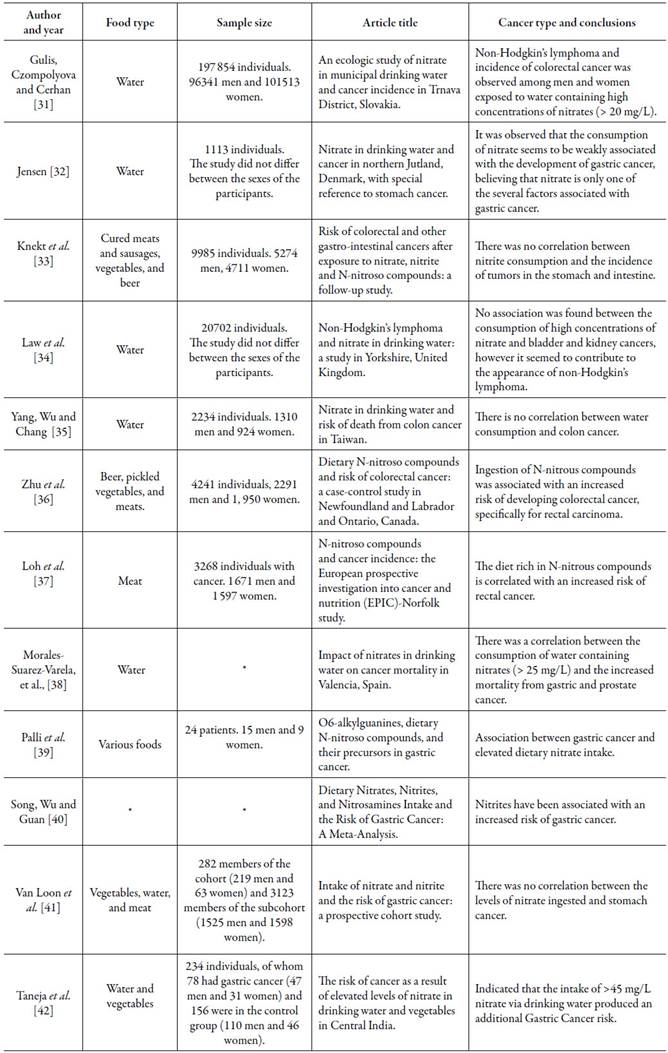
* No data.
Since 1992, studies have linked the consumption of nitrates and nitrites to cancer. It is known that cancer is a set of several neoplasms that can originate in any part of the body, having a multifactorial cause. In this perspective, the studies analyzed the relationship between the consumption of healing salts and the development of a variety of neoplasms and it was possible to observe that a direct association of the intake of these preservatives and the emergence of non-Hodgkin's lymphoma, gastric cancer, rectal cancer, and prostate cancer. However, some researchers have failed to prove this relationship [31-42]. This contradiction can be influenced by numerous factors, considering that the development of type of cancers can be related to several other population factors, such as age, gender, genetic predisposition, eating habits and lifestyle associated with smoking and consumption of alcohol, disregarding the rules and inspection of each country regarding the presence of these salts in the water and food offered to the population.
In studies in which correlations or an increased likelihood of neoplasms were observed, it was noted that gastric cancer has been the most cited of all, which is a worrying finding, since it is considered the sixth most common cancer in world and the third with the highest mortality rate [43, 44]. High mortality is associated with the fact that gastric cancer is usually silent, causing those affected to show signs and symptoms when the tumor is already quite developed. In these stages there is, for example, weight loss, constipation, vomiting and, in more advanced clinical situations, there is the presence of a palpable tumor mass [45]. A systematic review with meta-analysis, carried out on stomach cancer, showed that there is a statistically significant association between the high or moderate consumption of nitrates and the appearance of this type of cancer [46].
Colorectal cancer, in turn, covers tumors in the regions of the large intestine, mainly involving the colon, rectum and anus. Those affected usually have abdominal pain, bleeding in the stools, weight loss, anemia, and abdominal mass (tumor) [47], while non-Hodgkin's lymphoma is a type of cancer that originates in the lymphatic system, arising when a lymphocyte turns into a malignant cell, with the presence of water, fevers, night sweats, pruritus, and weight loss [48].
All cancers evidenced in the studies that are correlated with the consumption of NO2 -and NO3 -, can arise due to the excessive consumption of industrialized foods such as sausage, sausage, ham, bologna, salami, among others. In addition, it is important to highlight that, as already mentioned in this study, these foods are rich in nitrates and nitrites and in several studies, they present concentrations above those allowed by the laws of different countries, with the possibility of consuming foods rich in these preservatives, be directly related to the appearance of neoplasms [47].
In studies that observe a high consumption of nitrites and nitrites, there is a higher prevalence for the appearance of cancers when compared to populations where there is no consumption of such compounds. This finding has been proven in the laboratory through the quantification of nitrates in the urine and saliva of individuals affected by different types of cancers [49].
However, even though it is not completely clear, the general concern with prolonged exposure to nitrite and nitrate is due to the formation of nitrosamines, which are extremely carcinogenic nitrogen compounds [50]. In addition, it is important to show that nitrate is naturally eliminated from the body through urine and saliva, however nitrite tends to accumulate and contribute to the formation of N-nitrous compounds [51].
Nitrates are bioconverted to nitrites by reducing bacteria in the oral cavity, leading to increased production of free radicals. In addition, in the stomach, these nitrites are bioconverted to nitrosamines that are widely absorbed by the gastrointestinal tract, which can cause injury to several cells. Nitrosamines, once they come into contact with different cells from different tissues, can react with the DNA molecule, promoting the formation of adducts. But, for this, those compounds that do not undergo bioaccumulation need to be metabolized. Biotransformation starts from the hydroxylation of the carbon of the alkyl group, with the enzyme CYP2E1, as the main catalyst. Thus, there is the formation of an aldehyde or ketone and a primary nitrosamine, which tau-tomerizes to an alkyldiazohydroxide that can give rise to a diazonic ion, which takes up nucleophilic sites of DNA and RNA, with the formation of adducts and causing possible mutations (figure 3) [26, 51, 52, 53].

Figure 3 Mechanisms of adduct formation after ingesting nitrates and nitrites. I.Nitrates are bio-converted to nitrites, by reducing bacteria, in the oral cavity. II. In the stomach, nitrites are biocon-verted to nitrosamines. III.Nitrosamines undergo hydroxylation of the carbon of the alkyl group, with the enzyme CYP2E1 as the main catalyst. IV. There is formation of an aldehyde or ketone and a primary nitrosamine. V. Occurs tautomerization to an alkyldiazohydroxide. VI. Diazonic ion is formed. VII. The diazonic ion alkylates DNA nucleophilic sites. VIII. Adduct formation and possible mutations.
Another evidence that supports the hypothesis that these compounds are carcinogenic is the appearance of micronuclei in lymphocytes from individuals who excrete these nitrogen compounds in the urine. The presence of a micronucleus, by itself, indicates mutations and an increased risk of cancer [54].
In addition, nitrosamines, which can originate through nitrates and nitrites, have been classified in group 2A for toxic substances, indicating that there is indeed evidence of their carcinogenic potential in humans and rodents. Furthermore, the toxicity of nitrates and nitrites occurs differently for each person, since age, nutritional and immune status, environment, and frequency of ingestion of certain foods, may be associated with the appearance of cancers [2, 26, 53].
CONCLUSION
Nitrates and nitrites are important for the conservation and improvement of organo-leptic characteristics in various foods, and can be found in meat products, dairy products, fruits, and vegetables. For this reason, it is relevant to emphasize that it is already well documented in the literature that these compounds, when biotransformed, have a carcinogenic potential, so that it becomes interesting to avoid excessive consumption of these food inputs.
However, the different studies are still contradictory in relation to the results expressed. Thus, there is a lack of epidemiological studies that can characterize different populations and different neoplasms in relation to the consumption of these healing salts, making the data present in this research can be used to encourage the emergence of new research on this topic.