INTRODUCTION
The seed production in plant species with indeterminate growth habits (continuous flowering) and varying fruit maturity renders the mechanized harvesting processes. The harvest of fruits with different maturation levels produces seed lots of lower physiological quality (Pereira et al., 2014; Jorge et al., 2018; Silva et al., 2019; Santivañez et al., 2020). These seed quality differences indicate that the seed physiological responses can be standardized by selecting fruits of a similar ripening stage.
The seed’s physiological quality can also be improved after harvest when fruits are left to rest before the seed extraction and are properly dried for the preservation of their potential and health (Pereira et al., 2014; Figueiredo Neto et al., 2015; Silva et al., 2017; Nakada-Freitas et al., 2018; Santivañez, 2019). Consequently, proper postharvest treatment may allow early fruit harvest. Less time for seed production also decreases the field exposure of the fruits and seeds to weather variations, insect predation, and detrimental microorganisms.
Zucchini, courgette, or summer squash (Cucurbita pepo L.) is an example of a horticultural crop that presents diverse levels of fruit maturation in the same plant. During the postharvest period, seed maturation within the harvested fruits can improve physiological quality (Barroso et al., 2017; Melo Junior et al., 2019). Thus, this study evaluated the physiological quality of zucchini seeds from fruits that followed different postharvest resting periods.
MATERIAL AND METHODS
Growth conditions and plant material. Zucchini fruits were produced at São Paulo State University, campus Botucatu, in São Manuel city, Brazil, at 22°46’ S, 48°34’ W, about 740 m above sea level. According to the classification of Köppen, the climate is a Cfa type - temperate, warm, and humid (mesothermic) (Beck et al., 2018).
The soil was a typical Red Dystrophic Oxisol and presented the following characteristics in the 0-0.2 m soil layer: pH(CaCl2) = 5.4; organic matter = 19 g∙dm-3; P = 162 mg∙dm-3; H+Al = 20 mmolc∙dm-3; K = 2.8 mmolc∙dm-3; Ca = 36 mmolc∙dm-3; Mg = 8 mmolc∙dm-3; SB = 47 mmolc∙dm-3; CEC = 68 mmolc∙dm-3, and base saturation (V) = 70%. The soil preparation included plowing and harrowing activities, as indicated by Filgueira (2013). The application of limestone [high reactivity limestone (PRNT = 90%)] occurred 60 days before seedling transplanting to raise the base saturation to 80% and pH near 6.0. Fertilization was according to the recommendations (Raij et al.,1997).
Seeds of the Caserta cultivar were sown in polypropylene trays with 162 cells in the summer of 2018, containing a commercial substrate for vegetables (Carolina Soil®). Seedlings transplants to the field occurred 18 days after sowing. Each experimental parcel consisted of 30 zucchini plants spaced by 1 x 0.5 m in beds of 0.15 m height. Each treatment was replicated five times.
Field crop management. Drip irrigation was used to keep soil field capacity at about 60%. Dressing fertilization was divided into three applications, separated by a 14-day interval starting 14 days after transplanting. This fertilization corresponded to 120 kg∙ha-1 of each nitrogen (N) and K2O, applied as urea and potassium chloride, respectively. Regular cultural management is described by Filgueira (2013).
Zucchini harvest and seed treatments. Two zucchini fruits were left in each plant (60 fruits per experimental parcel); the other fruits were discarded. The fruits were harvested at 63 days after transplanting when they were yellowish. After harvest, the fruits rested for 0, 3, 6, 9, or 12 days on laboratory benches under ambient conditions at approximately 25°C and 80% relative humidity; the zucchini seeds were extracted after the resting periods.
The seeds were manually extracted by cutting the zucchini fruits longitudinally; the seeds were washed in running and distilled water. After washing, the seeds dried on clay dishes in a protected environment with an average temperature of 25°C. After drying, the seeds were placed in paper bags and maintained in a dry chamber (relative humidity: 40%; temperature: 20°C) until water content stabilized at 8%. The seeds were processed to remove the empty and damaged seeds.
Seed evaluations. The percent of processed seeds (cleaned seeds compared to the total processed seeds), the weight of 100 seeds, germination (%), first germination count, germination speed index (GSI), electrical conductivity, and the seed N and protein content were evaluated.
According to recommended methods (Brasil, 2009), the germination test was performed on processed seeds (5 replicates of 50 seeds each totaling 250 seeds per treatment). Seeds were placed on two moistened sheets of germination papers and covered with another sheet; water content in the moistened papers was 2.5 times the germination papers’ dry weight. The papers and seeds were rolled, kept in a plastic bag, and placed in a Biochemical Oxygen Demand (BOD) vertical chamber at 25°C.
The first count of normal seedlings was about four days after the start of the test (DAST). The final count was at 8 DAST, with seedlings having cotyledons, and the evaluations were daily until 8 DAST to obtain GSI, according to Maguire (1962).
Using the mass test method (Vieira & Dutra, 2006), electrical conductivity was estimated using five subsamples of 50 weighed seeds. Seeds were placed in plastic containers with 75 mL of deionized water to soak them for 8 hours at 30°C in a BOD chamber. The electrical conductivity reading was performed with a TEC-4MP meter (Tecnal®, Piracicaba, Brazil).
Seed nitrogen content was determined with the Kjeldahl method after the sulfuric digestion (Malavolta et al., 1997). Protein content was determined by the decomposition of proteins and other nitrogenous components with a Kjeldahl method in the presence of hot concentrated H2SO4; the results were multiplied by 6.25 to determine the total nitrogen (AOAC, 2019).
Statistical analysis. The observed results were subjected to the analysis of variance (ANOVA) and regression analysis using the SISVAR 5.3 (Ferreira, 2011) statistical software.
RESULTS AND DISCUSION
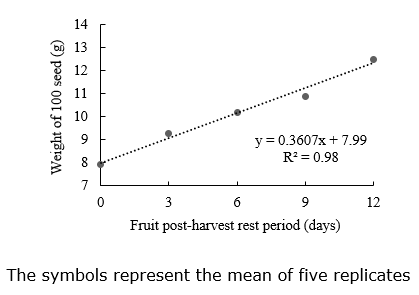
100 seeds’ weight. The zucchini fruit’s postharvest resting period (PHRP) affected 100 seeds’ weight, and it adjusted to a linear model. The linear model indicated that 100 seeds’ weight increases according to the period (days) the fruit remained at rest before extraction (Figure 1).
Even if fruits are ripe at harvest, the seed accumulation of carbohydrates, lipids, and proteins may occur during the resting period (Bareke, 2018), indicating the importance of the PHRP to the accumulation of seed reserves (Vidigal et al., 2006). Zucchini seeds with high weight present large reserves and are formed with great physiological quality (Carvalho & Nakagawa, 2012) since seeds with more reserves are available to the embryonic axis (Taiz et al., 2018).
Pumpkin (Cucurbita pepo L.) fruits with extended periods after anthesis also present great dry seed weight (Singh et al., 2020). However, when fruits are harvested immaturely, the appropriate PHRP is essential to increase seed physiological quality. In the present study, the assessed fruits were harvested when ripe but still exhibited an increased weight of 100 seeds, indicating that the ripe condition was not the end of the line for seeds to improve their biomass.
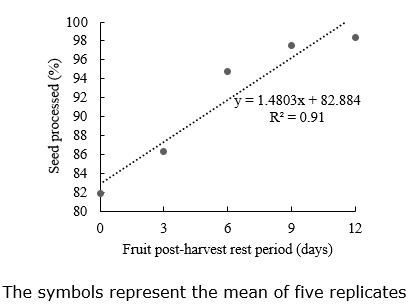
Percentage of seeds processed. The percentage of seeds processed (Figure 2) presented a linear adjustment regarding the PHRP when seeds were extracted shortly after harvesting through 12 days of PHRP. The empty and low-weight seeds were eliminated during the seed processing. The relationship between the cleaned seeds and the weight of 100 seeds was negative, indicating that a great 100 seeds weight generates fewer eliminated seeds during the processing. Thus, the PHRP increased the production of high-quality seeds, and fewer seeds were discarded during processing.
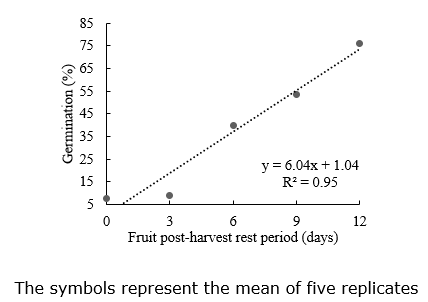
Seed germination. Seed germination was fitted to a linear model presented an estimated 1% germination in fruit without PHRP to 72.5% germination with 12 days of PHRP (Figure 3). When fruits were not left to rest, the low germination observed can be justified by the seed’s incomplete ripening inside the fruit. The postharvest rest was fundamental to the production and quality of the Caserta seed. The PHRP also increased germination in pumpkin (Figueiredo Neto et al., 2015; Singh et al., 2020) and zucchini (Marrocos et al., 2011).
Even at 12 days of PHRP, germination was estimated to be only at 72.5%. This relatively low result is probably due to some degree of seed dormancy, needing extended resting periods because the effect was linear and did not reach the maximum quality within the evaluated periods. Different aged fruit of a zucchini presented germination of only 46% in fruit at 50 days after anthesis (Sanches et al., 2017); this evaluation was in fruits without PHRP. The resting period is essential for some zucchini cultivars to reach higher germination rates. The physiological quality of species with fleshy fruit increases according to the degree of fruit maturity at harvest and postharvest resting period (Vidigal et al., 2006).
Another factor that affects the production of seeds in vegetables, especially Cucurbitaceae, is the number of fruits per plant (Cardoso et al., 2016). Still, in the present study, the number of fruits was limited to 2 per plant to reduce resource competition among fruits. For zucchini, the germination increased gradually during the PHRP up to 12 days and on, indicating that zucchini fruits need a longer PHRP (Figure 3).
Seed’s maturity in fleshy fruit coincides with a change in fruit color (Pereira et al., 2014); the yellowing of zucchini fruit is a reliable indicator of seed production with high physiological quality. Usually, fruit color is an excellent indicator of the seed’s physiological maturity stage (Melo Junior et al., 2019; Trancoso et al., 2021). The production of zucchini seeds with indeterminate growth is based on fruit color rather than on fruit age. The ideal zucchini fruit age to be harvested for seed production can vary according to climate, nutritional, and management conditions.
Research should be directed according to fruit color (Pereira et al., 2014) and should remain in the postharvest resting period to achieve maximum germination. The fruit weight, size, and water content can be used as harvest indicators, especially in fleshy fruit, aiming for maximum seed germination and vigor (Ellis, 2019; Rodrigues et al., 2020).
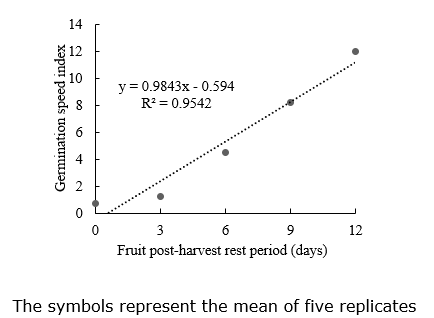
Germination speed index. The germination speed index data fitted to a linear distribution over PHRP (Figure 4). Besides age and postharvest resting period, other factors can affect the seed germination speed, such as crop management, depth of seed sowing, soil pH, light, temperature, and pollination, have a direct relation to the seed germination performance (Cardoso, 2005; Baskin & Baskin, 2018; Sarabi, 2019; Guo et al., 2020; Suriyasak et al., 2020; Travlos et al., 2020; Attri et al., 2021). Regarding the pollination in the present study, there were many pollinator insects, ensuring an adequate pollen supply for all zucchini flowers’ fertilization. Besides, only two fruits were left per plant, eliminating weaker fruits, which generally contain the least vigorous seeds.
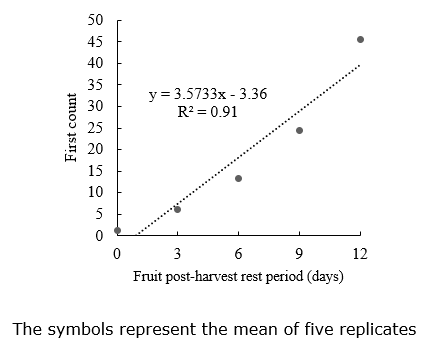
First germination count. For the first germination count, the data was fitted to a linear model, positively responding to the extension of the PHRP period (Figure 5). Increasing the postharvest resting period of the zucchini fruits also increases the seed vigor because the seeds continue developing inside the fruit, increasing the accumulation of reserves (Marcos Filho, 2015).
An adequate and efficient PHRP allows the anticipation of the harvest to protect the fruits and seeds from adverse climatic and phytosanitary factors. Also, early harvest reduces fruit exposure to adverse field conditions, possibly resulting in fruit predation and seed deterioration.
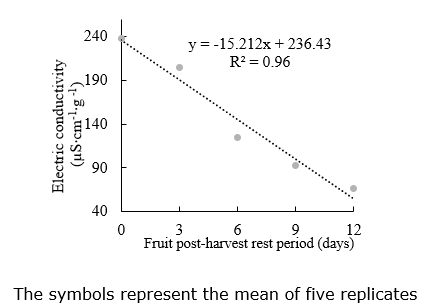
Electrical conductivity. The electrical conductivity data (Figure 6) presented a linear distribution of the data so that with increased PHRP, leachate decreased as fruit remained at rest for a more extended period. Higher electrical conductivity values mean lower membrane integrity, higher leachate output during seed imbibition, and lower seed quality (Pereira et al., 2014; Dayal et al., 2018).
In cucumber (Cucumis sativus), another species of the same plant family, the seed’s electrical conductivity as the age and PHRP increase (Nakada-Freitas et al., 2011). However, the PHRP does not affect tomato seed’s electrical conductivity in mature fruit (60 days after anthesis) because the cell membrane structure was already established (Vidigal et al., 2006).
Silva et al. (2015) identified that electrical conductivity increased and germination reduced in ten days of PHRP in pepper seeds ‘dedo de moça’ due to the beginning of the deterioration by late harvest. This was not observed in the present research, where fruits were not harvested after the ideal time.
Nitrogen (N) and protein contents. The N and protein contents (Figure 7) also fitted to a linear relation with the PHRP, where the N and protein contents increased every day the fruit was still in the PHRP. The highest N and protein levels were reached with 12 days of PHRP. Even if fruits are removed from the plant, there is still a translocation of N from fruit to seed and protein formation. Increasing protein content in seeds will consequently improve the weight and physiological quality of the seeds.
The complete seed maturation process coincides with high nutrient requirements; consequently, seeds with outstanding nutritional balance produce more vigorous seedlings because the nutrients stored in the seed will better supply the initial stages of germination (Carvalho and Nakagawa, 2012). Nitrogen, for instance, is one of the most accumulated nutrients in seeds because it is related to the high protein contents in seeds (Bu et al., 2018); therefore, the N levels in the zucchini seeds are essential for seed metabolism.
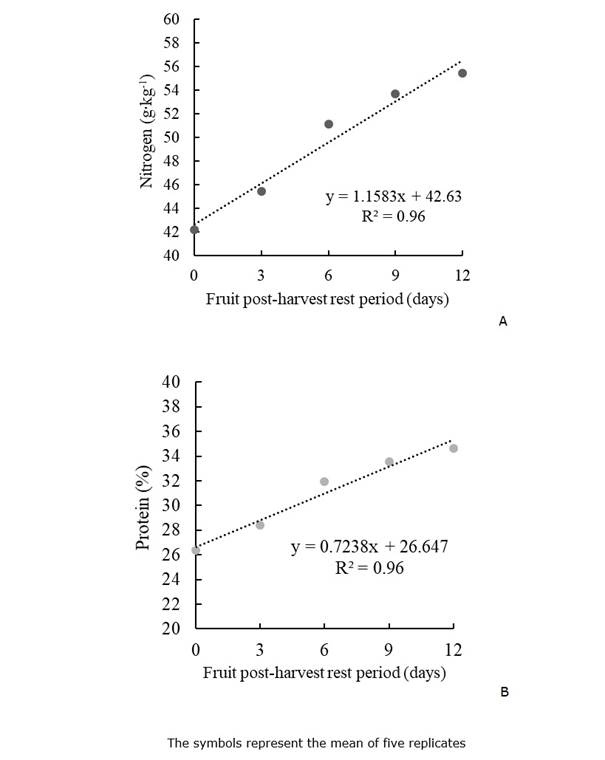
Figure 7 Nitrogen (A) and protein (B) content of zucchini seeds as a function of fruit postharvest rest period.
As the PRFC increased the weight of 100 zucchini seeds, the rate of processed seeds (cleaned seeds), germination, vigor, N, and protein contents in seeds also increased. These results indicate the advantages of the early harvest and PRFC for producing high-quality zucchini seeds (Dias et al., 2006; Vidigal et al., 2009; Pereira et al., 2014; Nakada-Freitas et al., 2018).
According to Jorge et al. (2018), the adequate management of PHRP can raise the production of homogeneous, high-quality zucchini seed lots. Vidigal et al. (2006) also pointed out this observation, where the number of harvests at different stages of tomato fruit maturation was reduced, improving the exploration of the seed maturation by the adequate use of the PHRP, reaching high physiological potential, and consequently more stable and productive seed lots.
CONCLUSIONS
The regression models indicate that the weight of 100 zucchini seeds, processed seeds, germination, seed vigour, the N, and protein contents increase as the resting period after harvest increases. Only the seed’s electrical conductivity decreased as days of postharvest rest increased.
The resting period of the zucchini fruits after harvest positively affected the production of homogeneous and high-quality seeds, potentially reducing the crop area occupancy and increasing the efficiency of the zucchini seed production.