Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista Colombiana de Entomología
Print version ISSN 0120-0488
Rev. Colomb. Entomol. vol.42 no.1 Bogotá Jan.,/June 2016
SECCIÓN BÁSICA / BASIC
ARTÍCULOS DE INVESTIGACIÓN / RESEARCH PAPER
Phorids associated with nests of Atta cephalotes (Hymenoptera: Formicidae) in a forest and a plantation
Fóridos asociados a nidos de Atta cephalotes (Hymenoptera: Formicidae) en bosque y plantación
Soraya UribeI; Brian V. BrownII; Guillermo CorreaIII; Adriana OrtizIV
IM.Sc. Sciences Master-Entomology, Faculty of Sciences, Universidad Nacional de Colombia-Sede Medellín, suribecelis@gmail.com
IIPh.D. Entomology Section, Natural History Museum of Los Angeles County, Los Angeles, USA. bbrown@nhm.org
IIIPh.D. Associate Professor, Faculty of Agrarian Sciences, Universidad Nacional de Colombia-Sede Medellín, gcorrea@unal.edu.co
IVD. Sc. Entomologist, Associate Proffesor, Faculty of Sciences, Universidad Nacional de Colombia-Sede Medellín, adortizr@unal.edu.co, corresponding author
ABSTRACT
The external portion of Atta cephalotes nests is composed of three areas: openings, trails, and cutting; where cutting and transporting leaves, sharing information, and defending the nest take place. The richness of the fauna of these areas is not only dependent on the interactions among ants, but also the accumulation of plant material and nest waste, which are exploited by flies of the family Phoridae. Traps with two different kinds of bait were used both during the day and at night to exploit common aspects of phorid fly biology and behavior, such as their attraction to live ants and refuse dumps, their use of visual and olfactory signals, and their perching behavior. Nests in both a citrus monoculture and a forest remnant were studied to evaluate whether environmental characteristics of the sites influence the presence of phorids. One parasitoid, Eibesfeldtphora attae, and 13 additional, mostly saprophagous, genera of phorids associated with A. cephalotes were collected in the nests. Specimens from 12 genera were identified in the forest remnant, most frequently Megaselia, Coniceromyia, and Synclinusa. Nine genera were identified in the citrus plantation, most frequently Dohrniphora and Megaselia. Using Analysis of Similarities (Anosim), significant differences (P = 0.002) in faunal composition were found between the forest remnant and the plantation, but no significant effect was detected in faunal composition between areas of the nest (P = 0.206), between baits (P = 0.956), or between periods (P = 0.603).
Key words: Tropical dry forest. Citrus. Interspecific interactions. Diptera. Leaf-cutter ants.
RESUMEN
La parte externa de los nidos de Atta cephalotes está conformada por tres áreas (bocas, trillas y áreas de forrajeo), en las cuales se desarrollan varias de las actividades ejecutadas por las hormigas, tales como corte y transporte de hojas, comunicación entre compañeras y defensa del nido. Estas áreas no solo son ricas por las interacciones entre las hormigas, sino porque allí se acumula material vegetal y desperdicios del nido que son explotados por dípteros de la familia Phoridae. El presente trabajo explora la distribución de los fóridos en las diferentes áreas del nido, en dos periodos diferentes (día y noche). Se utilizaron trampas con dos tipos de cebos, buscando explotar aspectos comunes de la biología y el comportamiento de los fóridos, tales como su atracción por las hormigas vivas y basureros, uso de señales visuales y olfativas, así como el comportamiento de posarse sobre una percha. Se seleccionaron nidos en áreas con ambientes diferentes: un monocultivo de cítricos y un remanente de bosque, para evaluar si las características de cada ambiente pueden influir en la presencia de fóridos. Se colectó un parasitoide de la especie Eibesfeldtphora attae, así como 13 géneros adicionales, asociados con A. cephalotes, siendo los de hábito saprófago los predominantes. En el remanente de bosque se identificaron especímenes de 12 géneros, siendo Megaselia, Coniceromyia y Synclinusa los más frecuentes. En la plantación de cítricos se identificaron 9 géneros, siendo Dohrniphora y Megaselia los más representativos. Usando análisis de similaridades (Anosim), se encontraron diferencias significativas (P = 0,002) entre el remanente de bosque y el cultivo, pero no se detectaron efectos significativos en la composición entre áreas del nido (P = 0,206), entre cebos ( P = 0,656), ni entre periodos (P = 0,603).
Palabras clave: Bosque seco tropical. Cítricos. Interacciones interespecíficas. Diptera. Hormigas cortadoras.
Introduction
Social insect nest architecture shows patterns that are specific to each species (Hölldobler and Wilson 1990). The nests of leaf-cutter ants of the genus Atta Fabricius possess highly specialized structures, including an internal and an external portion. The internal portion consists of a network of tunnels and chambers, which serve as shelter and protection against natural enemies and maintain the right microenvironmental conditions. This microenvironment promotes the survival of all the inhabitants of the colony, including the symbiont fungus Leucogaricus gongylophorus Singer (Möller), which is cultivated by the ants as a basis for their feeding (Moreira et al. 2004a).
The external portion of the nest is composed by three different areas, according to the definition given by Moreira et al. (2004b). A visible central area, which possesses several mounds (nest openings) that link the internal portion with the external one; some foraging trails, paths free of vegetation or any other obstacle, used by the ants for commuting; and the cutting areas, the places in which the ants forage. The foraging trails usually connect the openings and the cutting areas. Nevertheless, in some species like A. colombica (Guérin-Méneville, 1844) and A. mexicana (F. Smith), these trails also connect with external refuse dumps in which the ants place the waste material generated by the fungus and dead bodies of the ants or other intruder insects (Hart and Ratnieks 2002).
Each one of these areas (openings, trails and cutting) is modified by the activities of the ants and their intraspecific relationships, generating chemical, acoustic and visual signals. These signals may, in turn, be exploited by predators, and parasitoids of the ants (Feener and Brown 1997).
Parasitoid diptera of the family Phoridae that attack leaf-cutter ants are an example of the diversity of species exploiting this habitat (Elizalde and Folgarait 2012; Folgarait 2013, Disney and Bragança 2014). Eight genera have been reported as parasitoids of ants: Apocephalus Coquillett, Myrmosicarius Borgmeier, Neodohrniphora Malloch, Eibesfeldtphora Disney, Lucianaphora Disney, Allochaeta Borgmeier, Procliniella Borgmeier and Stenoneurellys Borgmeier (Elizalde 2009). Thirty species in eight genera have been related to the genus Acromyrmex Mayr; thirty-nine species in five genera have been described for the genus Atta. These species have been recorded mostly in Brazil and Argentina, where most of the research has been concentrated (Bragança 2011). This figures will probably increase with additional sampling in areas that have not been studied yet.
Search and attack strategies are diverse and characteristic of each phorid fly species, allowing improved resource utilization and coexistence of several species in the same habitat (Erthal and Tonhasca 2000; Tonhasca et al. 2001; Bragança et al. 2002). The selection of the hosts according to the body size might be necessary to ensure the development of the larva (Tonhasca et al. 2001; Bragança et al. 2003; Bragança and Medeiros 2006; Bragança et al. 2009). The place of oviposition in the body of the ant is another strategy that allows different species to use the same host (Brown 1999; Bragança et al. 2003). Selecting an area of the nest to stalk ants possibly allows multiple fly species to exploit the same nest without competing with each other (Bragança et al. 2003). Some phorids have even been detected in the refuse dumps, when these are located in the area outside the nest (Elizalde and Folgarait 2011; Elizalde and Folgarait 2012; Folgarait 2013).
Parasitoids are not the only phorids attracted to the nests of leaf-cutter ants. Several species of phorids have been collected on the external portion of the nest (Disney and Bragança 2014), possibly having been attracted to the debris generated by the ants, both inside and outside their nests. This material is rich in organic matter, making it attractive as food or an oviposition substrate.
The aim of this research was to evaluate the phorids associated with nests of Atta cephalotes (Linnaeus, 1758) leaf-cutter ants, comparing two different environments: a remnant of dry forest, where the most abundant plants belong to the families Euphorbiaceae J.F. Gmelin, 1777, Fabaceae Lindl (1836), Moraceae (Dumort., 1829) Link, 1831, Rubiaceae Juss (1789), Solanaceae Adans., 1763 and Rutaceae Durande, 1782, and a citrus orchard, mainly of Citrus sinensis L. We expected the forest remnant to yield a greater number of phorids, because of its greatest diversity of vegetation and the lack of disturbance associated with the management of the citrus crop. Each of these environments was assessed in two different periods: day and night, considering that there are some phorid species with nocturnal habits.
In some research on phorids associated with leaf-cutter ants, a manual vacuum cleaner is used as capture method, although this technique only allows the capture of ants or phorids that are flying over the nest. Trapping, however, offers the possibility of collecting a greater number of species that are present in the different areas of the nest. In addition, the vacuum cleaner method demands intensive researcher time to make collections.
Our methodology is based in part on methods used in monitoring Pseudacteon Coquillett, 1907 phorid flies that parasitize workers of fire ants, Solenopsis invicta Buren, 1972, in the United States (Puckett et al. 2007; Puckett et al. 2013). We designed two types of traps, that exploit some aspects of the behavior and biology of phorids, namely their attraction to live ants, attraction to aggregation or aggression pheromones, and their perching behavior (Puckett et al. 2007; Puckett et al. 2013).
Methods
This study was conducted from January to August 2011, in La Pintada municipality, department of Antioquia, Colombia (5°45'00"N 75°35'00"W), at 600 masl, with a bimodal rainfall distribution amounting to 1,000 mm annually. The average temperature is 27 °C and is classified as a tropical dry forest (TDF) (Holdridge 1987).
Two contrasting environments were selected. The first was located in a forest remnant in secondary succession, where the most abundant plant families were Euphorbiaceae, Fabaceae, Moraceae, Meliaceae, Rubiaceae, Rutaceae, and Solanaceae. The second environment was located in a citrus plantation.
A total of four samples were taken: two in the forest remnant and two in the citrus. Each of the samples included five active, mature nests of A. cephalotes, and considered three external areas: nest openings, main foraging trails, and cutting sites. Ten traps were placed in each area. Traps were built based on the model PTS used by Puckett et al. (2007). They consisted of a 15 cm-diameter expanded polystyrene plate, on top of which there was a round, 3 cm-diameter base of the same material, in which three equidistant toothpicks were inserted. The wooden toothpicks were coated with Tanglefoot® - a special glue for trapping insects.
Two different kinds of bait were evaluated into the traps as a way to increase the leaf-cutter ant activity to catch the phorids attention. The nature of the bait may influence on the type of pheromones and semiochemicals emitted by the ants (Brown and Feener 1991; Morehead and Feener 2000). Five plates in each area were supplied with citrus leaves; the five remaining ones were provided with refuse dumps from A. cephalotes nests (taken from artificial laboratory nests). Citrus leaves are very attractive for the ants, presumably because this substratum contains the nutrients required by the fungus cultivated by them. Refuse dumps are usually located in the inner portion of the A. cephalotes nests, so this bait could potentially elict a defensive response from the ants. Traps were collected every 12 hours to cover two periods: day (6:00 a.m.-6:00 p.m.) and night (6:00 p.m.-6:00 a.m.). These periods were included in the design because there are some species that show activity during the night (Disney 1994), such as Apocephalus attophilus Borgmeier, that exhibits nocturnal activity periods; whereas, some others require daylight to begin their activities, such as Eibesfeldtphora spp. (Bragança et al. 1998), Eibesfeldtphora curvinervis (Malloch) (Orr, 1992), and Megaselia nigra (Meigen, 1830) (Disney 1994).
Experimental design. A split-split-split-plot design was used. The levels of the environment factor (forest remnant and citrus plantation) were evaluated in the main plots (group of five nests) based on a randomized complete block design, where each block represented a sampling moment; the levels of the area factor (openings, foraging trails, and cutting sites) were evaluated in sub-plots, which were made up of the set of ten traps in each area associated with each nest; the levels of the bait factor (nest waste, citrus leaves) were evaluated in the sub-sub-plots (traps); the periods (day, night) represent the sub-sub-sub-plots.
Analysis of variance (ANOVA) was carried out using SAS® 9.1.3, with a significance level α = 0.05. Total number of phorids and genus-level composition were evaluated as response variables. When there were significant effects, the simple or principal effects of the means were analyzed, depending on each case, using the Least Significant Difference Test (LSD). The transformation 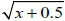 for the total number of phorids (response variable) was used in order to adjust errors to the normal distribution.
for the total number of phorids (response variable) was used in order to adjust errors to the normal distribution.
This analysis allows to contrast the phorid faunal composition between the different levels of the evaluated factors. The dissimilarities matrix was constructed based on the Bray-Curtis distance. Using the anosim function contained in the vegan package (Oksanen et al. 2015), an Analysis of Similarities was performed in R (R Core Team 2015).
Results
Inventory. Four hundred and twenty-nine individuals of the family Phoridae associated with the areas of the external part of A. cephalotes leaf-cutter ant nests were captured and classified into 14 genera (Table 1). Eibesfeldtphora attae Disney was the only parasitoid species found (but see discussion of Synclinusa Borgmeier, 1971, below), and it was located mainly in the forest remnant. Otherwise, Megaselia spp., Coniceromyia spp., and Synclinusa sp. were the predominant phorids captured in the forest remnant. Species of Dohrniphora Dahl and Megaselia Rondani were the most representative groups found in the citrus plantation.
Phorid location. The ANOVA for the number of phorids showed a significant interaction between the environment, area and period factors (P = 0.03). No differences were observed in the number of phorids between both environments evaluated for any of the combinations of the areas and period factors (Table 2). In the forest remnant, during the day, the number of phorids found in the traps placed at nest openings was statistically higher than those found on foraging trails and in cutting areas (Table 3). In the citrus plantation during the day, the number of phorids captured at nest openings was not statistically different from what was found on foraging trails and in cutting areas. However, the number of phorids found in cutting areas was statistically greater than what was found on foraging trails (Table 3). On the other hand, no significant effect of the type of bait or its interaction with other factors on the abundance of phorids was observed.

Contrasting the phorid generic composition by the Analysis of Similarities, a significant difference was found between the forest remnant and the citrus plantation (P = 0.002). No significant effect was detected between areas (P = 0.206), baits (P = 0.656), or periods (P = 0.603).
Discussion
Fourteen genera are reported in this research, that is, ten more than the found by Disney and Bragança (2014) in their investigation. Most of the genera reported in this study include saprophagous species (Table 1). This could mean that the nests of leaf-cutter ants have a variety of organic materials in decomposition, which attract these species. This explanation is in agreement with Rojas (1989), who found that the patches of decomposing organic at nest openings of A. mexicana promote the emergence of saprophagous insects that come to such places to feed and/or use this material as an oviposition site. Some species of the genera Apterophora Brues and Puliciphora Dahl have been collected in nests of A. sexdens Linnaeus, flying over the workers in the cutting areas and disturbed nests respectively (Disney and Bragança 2014). The female flies of the genus Puliciphora, which are wingless, are generally present in decomposition areas of dead or injured insects (Brown 2010).
The genera Dohrniphora and Megaselia were represented at all of the traps, areas and environments. It is difficult to generate conclusions in terms of gender, because both have many species and different habits. The life history of some species of the genus Dohrniphora have been described by Brown (2010), most of them with saprophagous habits, though there are also known species where the larvae are scavengers, fungivorous, predators, kleptoparasites, and parasitoids. Some species are associated with the nests of termites, ants and bees.
Megaselia were widespread, as they did not exhibit a marked tendency in any single evaluation site. This group is so vast that it probably has species that are favored by the environmental characteristics of both types of environments. The development of both adults and larva of Megaselia species is quite varied, which allows them to be successful in different types of ecosystems (Disney 1989).
The species of the genus Chonocephalus Wandolleck are characterized by sexual dimorphism; females are wingless. They have been found feeding on decaying plant material and carrion (Brown 2010).
Eurycnemis Borgmeier, Neopleurophora Brown, Synclinusa and Metopina-group are poorly studied genera. There are no data on their natural history (Brown 2010). Synclinusa spp. and Coniceromyia spp. have been reported for the first time associated with A. cephalotes nests. Although the natural history of these species is unknown, their abundant captures suggest that the environmental conditions of the forest remnant are the most suitable for the survival and successful reproduction of Synclinusa spp. and Coniceromyia spp. The rigid and highly sclerotized ovipositor of Synclinusa suggests parasitoid habits (Brown 2010).
Traps and baits used in this study were based on the designs proposed by Puckett et al. (2007), explicitly seeking to attract phorid parasitoids. Males of the parasitic phorid Apocephalus sp. were collected, species of this genus have been reported by Brown (2010) as parasitoids of ants. Myriophora Brown, are believed to be parasitoids of millipedes (Brown 2010), and all species of Pseudacteon are parasitoids of ants, including parasitoids of Solenopsis Westwood, 1841, Azteca Forel, 1878 and Crematogaster Lund, 1831 (Brown 2010). All specimens were collected in the forest remnant and no differences between nesting areas and type of bait were found.
Eibesfeldtphora attae, which is a parasitoid of A. cephalotes, was collected in both types of baits (10 specimens in leaves and 10 specimens in refuse dumps), indicating that this species shows no preference for bait. A greater number of specimens was collected at the openings of the nest (13 captures) compared with the cutting and the trails. This result is possibly related to the aggregation of ants in the nest openings, which stimulates the presence of the parasitoid and its attack behavior. Brown et al. (2012) describe the attack behavior and oviposition site of E. breviloba Brown and E. digitata Brown. Females in both species stalk ants from their back or sides and it seems that they introduce the ovipositor behind their head. Bragança et al. (2002) suggest a different behavior for E. erthali (Brown) and E. bragancai (Brown), whose females introduce the ovipositor in the ant gaster.
The environment (crop, forest remnant) did make a difference in the presence of parasitoids. Twenty specimens were collected in the forest remnant, while only one specimen was captured in the citrus crop. This result is consistent with the one reported by Almeida et al. (2008), who found a significant decrease in the number of phorid parasitoids in border areas compared to a mature forest. This decrease could be related to the microclimatic changes between these environments, affecting the survival of the parasitoids. Pesquero et al. (2010) and Gomes et al. (2013) reported similar results with other species of the genus Eibesfeldtphora, which could be related to effects of the environmental change between these environments, causing a reduction in the nutritional supply for the phorids in the crop environment.
Conclusions
This is the first inventory of phorids associated with the A. cephalotes nests accomplished in Colombia. Fourteen genera were reported, showing a high diversity of species that have not been studied yet. The finding of parasitoid species in the forest remnant opens the possibility to future studies in biological control of leaf-cutter ants and encourages producers to maintain this type of environment to promote the presence of parasitoid species. This research provides the first Colombian report of the phorids of the species Eibesfeldtphora attae, a specific parasitoid of the leaf-cutter ants of the genus Atta.
Acknowledgements
We thank Vicerrectoría at Universidad Nacional de Colombia-Sede Medellín for funding this study (code 90201016). We thank CORANTIOQUIA for granting the permit for scientific research in biological diversity (code 4857), and Monteloro y Agrotúnez S. A. farms, for allowing us to conduct our field work on their property.
Literature cited
ALMEIDA, W. R.; WIRTH, R.; LEAL, I. R. 2008. Edge-mediated reduction of phorid parasitism on leaf-cutting ants in a Brazilian Atlantic forest. Entomologia Experimentalis et Applicata 129: 251-257. [ Links ]
BRAGANÇA, M. A. L.; TONHASCA, A. J.; DELLA LUCIA, T. M. C. 1998. Reduction in the foraging activity of the leaf-cutting ant Atta sexdens caused by the phorid Neodohrniphora sp. Entomologia Experimentalis et Applicata 89: 305-311. [ Links ]
BRAGANÇA, M. A. L.; TONHASCA, A. J.; MOREIRA, D. D. O. 2002. Parasitism characteristics of two phorid fly species in relation to their host, the leaf-cutting ant Atta laevigata (Smith) (Hymenoptera: Formicidae). Neotropical Entomology 31: 241-244. [ Links ]
BRAGANÇA, M. A. L.; DELLA LUCIA, T. M. C.; TONHASCA, A. J. 2003. First record of phorid parasitoids (Diptera: Phoridae) of the leaf-cutting ant Atta bisphaerica Forel (Hymenoptera: Formicidae). Neotropical Entomology 32: 169-171. [ Links ]
BRAGANÇA, M. A. L.; MEDEIROS, Z. C. S. 2006. Ocorrência e características biológicas de forídeos parasitóides (Diptera: Phoridae) da saúva Atta laevigata (Smith) (Hymenoptera: Formicidae) em Porto Nacional, TO. Neotropical Entomology 35(3): 408-411. [ Links ]
BRAGANÇA, M. A. L.; TONHASCA, A. JR.; DELLA LUCIA, T. M. C. 2009. Características biológicas e comportamentais de Neodohrniphora elongata Brown (Diptera, Phoridae), um parasitóide da saúva Atta sexdens rubropilosa Forel (Hymenoptera, Formicidae). Revista Brasileira de Entomologia 53 (4): 600-606. [ Links ]
BRAGANÇA, M. A. L. 2011. "Parasitoides de formigas cortadeiras," in Formigas - Cortadeiras: da bioecologia ao manejo, T.M. castro Della Lucia, Ed., pp. 321-343, Universidade Federal de Viçosa, Brasil. [ Links ]
BROWN, B. V.; FEENER, D. H., JR. 1991. Behavior and host location cues of Apocephalus paraponerae (Diptera: Phoridae), a parasitoid of the giant tropical ant Paraponera clavata (Hymenoptera: Formicidae). Biotropica 23: 182-187. [ Links ]
BROWN, B. V. 1999. Differential host use by neotropical phorid flies (Diptera: Phoridae) that are parasitoids of ants (Hymenoptera: Formicidae). Sociobiology 33: 95-103. [ Links ]
BROWN, B. V. 2010. Phoridae. pp. 725-761. In: Brown, B. V.; Borkent, A.; Cumming, Woodley, J. M., N. E.; Wood, D. M.; Zumbado, M. A. (Eds.). Manual of Central American Diptera, Volume 2. NRC Research Press, Ottawa. [ Links ]
BROWN, B. V.; BRAGANCA, M. A. L.; GOMES, D. S.; QUEIROS, J. M; TEIXEIRA, M. C. 2012. Parasitoid phorid flies (Diptera: Phoridae) from the threatened leafcutter ant Atta robusta Borgmeier (Hymenoptera: Formicidae). Zootaxa 3385: 33-38. [ Links ]
DISNEY, R. H. L. 1989. Scuttle flies (Phoridae: Megaselia). Handbooks for the identification of British Insects 10 (8). Royal Entomological Society of London. 155p. [ Links ]
DISNEY, R. H. L. 1994. Scuttle flies: the Phoridae. Chapman and Hall, London, UK. 467 p. [ Links ]
DISNEY, R. H. L.; BRAGANÇA, M. A. L. 2014. New Records, Including a New Species, of Scuttle Flies (Diptera: Phoridae) Associated with Leaf Cutter Ants (Hymenoptera: Formicidae) in Brazil. Sociobiology 61 (3): 341-344. [ Links ]
ELIZALDE, L. 2009. Biogeografía y comunidades de fóridos parasitoides de hormigas cortadoras de hojas. Universidad Nacional de Quilmes. [ Links ]
ELIZALDE, L.; FOLGARAIT, P. J. 2011. Biological attributes of Argentinian phorid parasitoids (Insecta: Diptera: Phoridae) of leaf-cutting ants, Acromyrmex and Atta. Journal of Natural History 45: 2701-2723. [ Links ]
ELIZALDE, L.; FOLGARAIT, P. J. 2012. Behavioral strategies of phorid parasitoids and responses of their hosts, the leaf-cutting ants. Journal of Insect Science 12: 1-26. [ Links ]
ERTHAL, M. JR.; TONHASCA, A. JR. 2000. Biology and oviposition behavior of the phorid Apocephalus attophilus and the response of its host, the leaf-cutting ant Atta laevigata. Entomologia Experimentalis et Applicata 95: 71-75. [ Links ]
FEENER, D. H.; BROWN, B. V. 1997. Diptera as parasitoids. Annual Review of Entomology 42: 73-97. [ Links ]
FOLGARAIT, P. J. 2013. Leaf-cutter ant parasitoids: Current knowledge. Psyche (New York), 10 p. [ Links ]
GOMES, D. S.; ELIZALDE, L.; QUEIROZ, J. M. 2013. Parasitoids of the endangered leafcutter ant Atta robusta Borgmeier in urban and natural areas. Revista Brasileira de Entomologia 57: 335-339. [ Links ]
HART, A. G.; RATNIEKS, F. L. W. 2002. Waste management in the leaf-cutting ant Atta colombica. Behavioral Ecology 13 (2): 224-231. [ Links ]
HOLDRIDGE, L. R. 1987. Ecología basada en zonas de vida. Tercera edición. Instituto Interamericano de Cooperación para la Agricultura (IICA), San Jose, Costa Rica. 266 p. [ Links ]
HÖLLDOBLER, B.; WILSON, E. O. 1990. The ants. Cambridge, M.A: Belknap Press. 732 p. [ Links ]
MOREIRA, A.; FORTI, L. C.; ANDRADE, A. P.; BOARETTO, M. A.; LOPES, J. 2004a. Nest architecture of Atta laevigata (F. Smith, 1858) (Hymenoptera: Formicidae). Studies on Neotropical Fauna and Environment 39 (2): 109-116. [ Links ]
MOREIRA, A.; FORTI, L. C.; BOARETTO, M. A. C.; ANDRADE, A. P. P.; LOPES, J. F. S. ; RAMOS, V. M. 2004b. External and internal structure of Atta bisphaerica Forel (Hymenoptera: Formicidae) nests. Journal of Applied Entomology 128: 204-211. [ Links ]
MOREHEAD, S. A.; FEENER, D. H. JR. 2000. Visual and chemical cues used in host location and acceptance by a dipteran parasitoid. Journal of Insect Behavior 13 (4): 613-625. [ Links ]
ORR, M. R. 1992. Parasitic flies (Diptera: Phoridae) influence foraging rhythms and caste division of labor in the leaf-cutter ant, Atta cephalotes (Hymenoptera: Formicidae). Behavioral Ecology and Sociobiology 30: 395-402. [ Links ]
OKSANEN, J.; BLANCHET, G. F.; KINDT, R.; LEGENDRE, P.; MINCHIN, P.; O'HARA, R. B.; SIMPSON, G. L.; SOLYMOS, P.; STEVENS, M. H. H.; WAGNER, H. 2015. vegan: Community Ecology Package. R package version 2.2-1. Available in: http://CRAN.R-project.org/package=vegan. Review date: [05-12-2015]. [ Links ]
PESQUERO, M. A.; BESSA, L. A.; SILVA, H. C. M.; SILVA, L. D. C.; ARRUDA, F. V. 2010. Influência ambiental na taxa de parasitismo (Diptera: Phoridae) de Atta laevigata e Atta sexdens (Hymenoptera: Formicidae). Revista de Biologia Neotropical 7(2): 45-48. [ Links ]
PUCKETT, R. T.; CALIXTO, A.; BARR, C. L.; HARRIS, M. 2007. Sticky traps for monitoring Pseudacteon parasitoids of Solenopsis fire ants. Environmental Entomology 36 (3): 584-588. [ Links ]
PUCKETT, R. T.; CALIXTO, A.; SMITH, J.; JOHNSON, J.; REED, J. 2013. Effectiveness comparison of multiple sticky-trap configurations for sampling Pseudacteon spp. phorid flies. Environmental Entomology 42 (4): 763-769. [ Links ]
ROJAS, P. 1989. Entomofauna asociada a los detritos de Atta mexicana (F. Smith) (Hymenoptera: Formicidae) en una zona árida del centro de México, Acta Zoológica Mexicana 33: 1-51. [ Links ]
R CORE TEAM. 2015. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL http://www.R-project.org/. [ Links ]
TONHASCA, A. JR.; BRAGANÇA, M. A. L.; ERTHAL, M. JR. 2001. Parasitism and biology of Myrmosicarius grandicornis (Diptera, Phoridae) in relationship to its host, the leaf-cutting ant Atta sexdens (Hymenoptera, Formicidae). Insectes Sociaux 48: 154-158. [ Links ]
Received: 14-May-2014
Accepted: 30-Mar-2015














