Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista Colombiana de Entomología
Print version ISSN 0120-0488
Rev. Colomb. Entomol. v.42 n.2 Bogotá 2016
Sección Control / Control
Effects of Capsicum baccatum and C. frutescens against Atta cephalotes (Hymenoptera: Formicidae) and the symbiotic fungus Leucoagaricus gongylophorusEfectos de Capsicum baccatum y C. frutescens sobre Atta cephalotes (Hymenoptera: Formicidae) y el hongo simbionte Leucoagaricus gongylophorus
TATIANA LOBO-ECHEVERRI1,2, LINA CRISTINA SALAZAR1,3, ALEJANDRA HERNÁNDEZ4 y ADRIANA ORTIZ-REYES1,5
1 Facultad de Ciencias, Universidad Nacional de Colombia, Sede Medellín. Calle 59 A 63-20, 21-326, Medellín, Colombia.
2 Associate Professor. Ph. D. Escuela de Química, tloboech@unal.edu.co, corresponding author.
3 Student, Maestría en Ciencias-Química, lcsalazarleon@gmail.com.
4 Student, Instituto de Biología, Facultad de Ciencias Exactas y Naturales, Universidad de Antioquia. Calle 67 Número 53-108, Medellín, Colombia, ale8469@gmail.com.
5 Associate Professor. Ph. D. Escuela de Biociencias, adortizr@unal.edu.co.
ABSTRACT
Leaf cutter ants are considered to be a major pest in the Neotropics, due to the considerable economic losses they cause by cutting large amounts of plant material to cultivate their symbiotic fungus. Their control is mainly achieved through synthetic products with adverse consequences to the environment and human health. In search for alternatives, the ethanolic extracts of leaves of Capsicum baccatum and C. frutescens (Solanaceae), were evaluated against medium size leaf cutter ant Atta cephalotes, and its symbiotic fungus Leucoagaricus gongylophorus. The results were promising as both plant extracts exhibited a combination of insecticidal and antifungal activity when evaluated at concentrations of 0.10, 0.25, and 0.50 % w/v. Thus, C. baccatum was shown to be the most promising as an insecticidal while, C. frutescens presented a better antifungal activity at high concentrations. Since secondary metabolites present in plants are responsible for their bioactivity, preliminary phytochemical tests and gas chromatography coupled with mass spectrometry (GCMS) of both species were carried out. In qualitative metabolite analysis, major groups detected were alkaloids, terpenoids and phenols, which are the compounds cited with the highest frequency in the management of the leaf cutter ant. Some nuclei were confirmed by GCMS, such as caryophyllene and the alkaloid conhidrine detected in C. baccatum, and precursors of capsaicin in C. frutescens. In this way, both species are considered promising leads for a more efficient integrated management of the leaf cutter ants.
Key words: Antifungal activity. Insecticide. Leaf cutter ants. Secondary metabolites.
RESUMEN
Las hormigas cortadoras de hojas son consideradas una plaga mayor en el Neotrópico, debido a las pérdidas económicas considerables que causan al cortar grandes cantidades de material vegetal, para cultivar su hongo simbionte. Su control se da principalmente a través de productos sintéticos con consecuencias adversas para el ambiente y la salud humana. En busca de alternativas, los extractos etanólicos de las hojas de Capsicum baccatum y C. frutescens (Solanaceae), fueron evaluados contra la hormiga cortadora de hojas Atta cephalotes y su hongo simbionte Leucoagaricus gongylophorus. Los resultados fueron prometedores, ya que ambos extractos exhibieron una combinación de actividad insecticida y antifúngica cuando fueron evaluados a concentraciones de 0,10, 0,25 y 0,50 % de m/v. De tal manera, C. baccatum se destacó por su actividad insecticida, mientras que C. frutescens presentó una mayor actividad antifúngica a altas concentraciones. Como los metabolitos secundarios presentes en plantas son responsables por su bioactividad, se llevaron a cabo evaluaciones fitoquímicas preliminares y cromatografía de gases acoplada a espectroscopia de masas (CGEM) para ambas especies. En el análisis cualitativo de metabolitos los grupos mayoritarios detectados fueron alcaloides, terpenoides y fenoles, los cuales son los compuestos que más frecuentemente citan en el manejo de la hormiga cortadora de hojas. Algunos de estos núcleos fueron confirmados por CGEM, tales como el cariofileno y el alcaloide conhidrina en C. baccatum y precursores de capsaicina en C. frutescens. De esta manera, ambas especies son consideradas como una alternativa prometedora para un manejo integrado de la hormiga cortadora de hojas más eficiente.
Palabras clave: Actividad antifúngica. Insecticida. Hormigas cortadoras de hojas. Metabolitos secundarios.
Introduction
Leaf cutter ants of the genus Atta and Acromyrmex, classified into the Attini tribe (Hymenoptera: Formicidae), are considered pests in the Neotropics due to the amount of leaves they cut to cultivate the symbiotic fungus Leucoagaricus gongylophorus (Möller) Singer (Chaves 2006). In the region of Colombia, four species of Atta and eight of Acromyrmex have been recognized (Fernández et al. 2015), from which Atta cephalotes has the widest distribution predominating in Antioquia and Valle del Cauca (Ortiz and Guzman 2007). In recent years, the foraging activity of the leaf cutter ants has intensified as deforestation and monoculture farming has increased (del Toro et al. 2009; Dohm et al. 2011). Quantitative data on the biomass consumption by leafcutter ants is lacking and the economic losses in areas of anthropogenic activity are difficult to estimate (Della Lucia 2003). The control of the leaf cutter ant has been a challenge due to a series of adaptations, such as its social organization, hygiene, and complex nest structure (Della Lucia et al. 2014). Such adaptations along with their few natural enemies with little impact on their populations make these insects unique, so management should differ from those applied for other pests (Herrera and Valenciaga 2011). Although alternative methods of ant control have been studied, synthetic agrochemicals prevail in this case (Della Lucia et al. 2014). Chemicals like malathion, perchlordecone, heptachlor, chlorpyrifos, fipronil or sulfuramide have been applied liquid, by thermal fogging or in granular baits (Paoletti and Pimentel 2000, Forti et al. 2007, de Britto et al. 2016). Due to the lack of specificity, these highly persistent agrochemicals affect beneficial insects, mammals, and have generated resistance among ant populations, with adverse consequences to the environment and to human health (Rauh et al. 2012; dos Santos et al. 2016). In view of the prohibition or restriction of some synthetic products, their substitution for other effective and selective substances against the leaf cutter ants is essential.
Various studies describe alternative methods to control of the leaf cutter ant, some reported the use of antagonist fungus (Trichoderma viride Pers., T. lignorum (Tode) Harz), and enthomopathogens (Metarhizium anisopliae (Metschn.) Sorokin, Beauveria bassiana Vuill, Paecilomyces sp.), for species of Atta and Acromyrmex (López and Orduz 2003; Montoya-Lerma et al. 2012). Plant extracts have also been evaluated for their activity against the ant or their antifungal potential against L. gongylophorus. Due to their symbiotic relationship, management can be considered at an antifungal or insecticidal level. Thus some plant extracts or fractions have promising activity only against the ants such as species of Citrus (Rutaceae) that presented toxicity in topic applications (Fernandes et al. 2002), or fractions derived from the leaves of tomato (Lycopersicum esculentum Mill, Solanaceae) with repellency in a laboratory colony of A. cephalotes (Serna and Correa 2003). Whereas other species have shown activity only as antifungals, for instance the inhibition exhibited by Simarouba versicolor A. St. Hil. and S. guianensis (Simaroubaceae) (Zavan 2005; Peñaflor et al. 2009).
Alternatively, some plants extracts or compounds have shown bioactivity against both organisms, the ant and its symbiotic fungus, among which the Lamiaceae and Apiaceae families have been the most promising families (Boulogne et al. 2012). Species of other plant families also had effects in laboratory experiments on the symbiotic fungi and on A. cephalotes. A significant study of 89 plant species native to Argentina showed that 13.5 % of the plants inhibited the foraging activity of Acromyrmex lundi (Guerin) and 12.3 % inhibited the fungal growth. Aristolochia argentina and Fluorensia oolepsis presented both activities (Diaz Napal et al. 2015). Related laboratory studies against Atta species and L. gongylophorus identified Canavalia ensiformes L. (Fabaceae), Tithonia diversifolia (Hemsl.) A. Gray (Asteraceae) (Aubad 2010; Valderrama-Eslava et al. 2009), Cedrela fissiles Vell. (Meliaceae) (Bueno et al. 2005), Virola sebifera L. (Myristicaceae) (Pangocca et al. 1996), Sesamum indicum L. (Pedaliaceae) (Bueno et al. 2005) as some of the most promising leads. As for the plant-derived compounds, 20 metabolites with insecticidal and fungicidal activities have been identified, where terpenoids have been the most promising group (Boulogne et al. 2012). The sesquiterpenes caryophyllene, caryophyllene epoxide, and nerolidol, reported in extracts of Hymenaea coubaril L. (Caespalpiniaceae), Melampodium divaricatum (L.C. Rich) DC (Asteraceae), and Vismia baccifera (L.) Triana & Planch (Clusiaceae) with repellent and antifungal activities (Howard et al. 1988).
While many studies have proven the efficacy of plantderived products (extracts and compounds) in laboratory experiments, follow up studies have not been carried out with the promising leads. Chemical control is the only method with practical technology (de Britto et al. 2016) consequently the problem of controlling species of Atta in a sustainable way, still persists. In contrast, farmers and indigenous communities in Colombia have traditional knowledge about the use of leaves and plant resins to control the leaf-cutting ants (Agudelo 2007). Unfortunately, in many communities this knowledge has decreased and as homogenous monocultures have been implemented, traditional practices have been replaced by the use of commercial synthetic products. Alternatively, the Solanaceae is one of the families with the most poisonous plants (Lee 2006). Extracts of different plant parts and compounds have been widely use as pesticides (Chowanski et al. 2016). The purpose of this study was to test the efficacy of the traditional use of two Capsicum species (Aubad 2010), against A. cephalotes and its antifungal activity against L. gongylophorus. As an initial step in the standardization of the whole plant extract, detection of secondary metabolites was performed in order to characterize the major metabolites that could be implicated in the bioactivity.
Materials and methods
Plant material. Fruits of Capsicum baccatum L. and C. frutescens L. were bought at the local market and cultivated between May to September at full sun conditions, in composted soil, at 2120 m.s.l. Once plants grew and where at a young reproductive stage, 1 kg of leaves, were collected for laboratory analysis. For the correct identification of each plant, voucher samples were deposited at the Herbarium of the National University, Medellin (MEDEL), codified as Atta- 04 (C. frutescens) and Atta-12 (C. baccatum). Samples were identified by Professor Mauricio Sánchez (Departamento de Ciencias Forestales, Universidad Nacional de Colombia-sede Medellín).
Plant extraction. Leaves of C. baccatum and C. frutescens separately were dried at room temperature and extracted with 90 % ethanol overnight (1L x 100 g), solvent was drained. This process was repeated two more times for an exhaustive extraction. The combined ethanol extract was filtered and concentrated under reduced pressure, using a rotary evaporator at a temperature below 40 ºC. The resultant ethanolic extract was mixed with distilled water to a 10 % solution and defatted with hexane. Subsequently, the ethanolaqueous extract was extracted three times with an equal volume of chloroform, to afford a chloroform soluble extract. The resulting fraction was completely dried under vacuum for further analysis.
Bioassays. The ants used in the bioassays came from three artificial nests from colonies collected in Barbosa (Antioquia) at 1300 m.s.l., which were established in the laboratory in 2010 (Permits under resolution 15046 Corantioquia). The artificial colonies were kept at a temperature of 23.45 °C, and a relative humidity of about 62.30 %, in partial darkness with the weekly administration of leaves of Acalypha wilkesiana Mull. A (Euphorbiaceae), Citrus sp. (Rutaceae), and Terminalia catappa L. (Combretaceae) (Ortiz 1998). The concentrations for the biological testing were based on an average of previous bioassays with plant extracts of other studies, and the concentrations of pesticides (Loeck and Gusmão 1998; Serna and Correa 2003).
Insecticidal activity. For each treatment, 50 medium-sized A. cephalotes workers were picked from the artificial nests. The ant's size was established based on the protocols of Giraldo (2008). To evaluate the insecticidal bioactivity, the ants were distributed randomly in groups of 10 ants in 5 petri dishes (Oliveira 2006). The two Capsicum extracts were incorporated into a solid diet (Bueno et al. 1997), at concentrations of 0.1, 0.25, and 0.5 % (w/v). Cellulose was added as a non-nutritive ingredient at concentrations of 150, 375, and 600 mg (for 0.1, 0.25, and 0.5 % w/v, respectively), to disperse the solid extract that was not soluble in the diet media (Aubad 2010). The solid diet was changed on a daily basis for each treatment, retiring the diet of the day before when it was eaten at least more than half. As negative controls the same diet was offered to the same number of ants (50 individuals distributed in groups of 10 in 5 petri dishes) without the incorporation of the extracts, but with highest concentration of cellulose used to disperse the extracts at 0.5 % (w/v) and another group only with the solid diet. As positive control, Lorsban (Dow Agro Sciences) was added to the diet (Aubad 2010). Assays were replicated three times and the number of dead ants was registered daily during five days to calculate the results based on the Abbott's correction for natural mortality (Abbot 1987).
Antifungal activity. For the antifungal assay, samples of L. gongylophorus were taken directly from the three artificial nests and inoculated in PDA (potato dextrose agar), with lactic acid at a pH of 4.5 to avoid bacterial contamination. The in vitro cultures of the fungi were kept in the dark at 26 ºC with a relative humidity of 79 %. For the bioassay, the fungus was cultivated with the dried extracts, which were dispersed with cellulose in the culture media at concentrations of 0.1, 0.25, and 0.5 % (w/v). The negative controls, included the fungus cultivated in the PDA and cellulose without any extract and the fungus cultivated only in PDA (Pagnocca et al. 1996). No positive control was used since there is not a standard product with action against L. gongylophorus. For each treatment, five repetitions were done. The percent of inhibition was calculated after measuring the fungus growth according to the length of four fixed perpendicular radius, measuring always the same radius (Maya 2002). Fungal growth was registered for five weeks and compared to the negative control to calculate the inhibition percentage.
Data analysis. For the results obtained in the insecticidal bioassay of A. cephalotes, the percentage of death ants caused by the treatments were calculated using the Abbott's corrected mortality percentage formula:

Where Co is number of live control ants after treatment, T number of live ants in the treatment (Puntener 1981).
After calculating the percentage of mortality with Abbott's formula, the probability of death of ant individuals was assessed by applying different doses of the concentration of the extracts of C. frutescens and C. baccatum. The probability of death of the ants was calculated by means of a logistic regression with binomial errors (PROBIT analysis). Dead ants were treated as success and alive ants as failures. To assess the probability of death we used the log link function that expresses the probability of death (p) as follows:

Where x is the concentration of plant extract employed and a and b the parameters of the model (Crawley 2012).
Based on the fitted logistic model, the doses of plant concentration needed to kill 50 %, 90 %, or 95 % of the individuals of each species, were estimated. The analyses were performed using the R software version 3.01 (R Core Team 2014). We used the dose.p function available in the library MASS to calculate the doses needed to kill a predefined percentage of individuals.
For the antifungal activity, the percentage inhibition was calculated based on the fungal growth (colony diameter) of the control samples as reference, according to the formula:

Where C is the colony diameter (mm) of the control and T the colony diameter (mm) of the test plate.
To identify the variation at different doses of the antifungal activity, a one way analysis of variance (ANOVA) of the percentage inhibition at each dose, was conducted. When significant differences were detected (α ≤ 0.05), a Tukey’s range test, with a confidence level of 95 %, was done to establish the differences at each level of activity. The analyses were performed using the R software version 3.01 (R Core Team 2014).
Phytochemical analysis. Alkaloids: The dry ethanolic extracts (4 mL) of C. baccatum and C. frutescens were stirred with 4 mL of 1 % HCl on steam bath, this was filtered and separated in 4 tubes (1 mL each). Each tube was analyzed by four different reagents, namely, Dragendorff, Mayer, Valser and ammonium Reineckate. Change of color or turbidity of the resulting precipitate was taken as evidence of the presence of alkaloids (Kavit et al. 2013).
Anthraquinones: The Bornträger reaction was carried out, starting with a basic hydrolysis with KOH (5 %) to the ethanolic extracts (5 mL), which was then was acidified with acetic acid and re-extracted with benzene. The resultant extraction was stirred with 2.5 mL of NaOH (10 %). The red coloration in the alkaline phase was considered positive (Yusuf et al. 2014).
Cardiotonic glicosides: Keller-kiliani test was performed, in which 1mL of glacial acetic acid and 1-2 drops of FeCl3 was added followed by 1mL of concentrated H2SO4 to 2 mL of the extract of each Capsicum species. Green blue color indicated the presence of cardiac glycosides (Parekh and Chanda 2007).
Coumarins: the Lock test was carried out taking 2 mL of the ethanolic extracts that were covered with filter paper impregnated with a diluted NaOH solution in a steam bath. The filter paper was removed and the extract was analyzed under UV light. A yellow fluorescence was an indication of the presence of coumarins (Lock 1988).
Saponins: The dried extracts (2 mL) were re-dissolved in 5 ml of distilled water, then shaked well and evaluated for its frothing persistence (Kavit et al. 2013).
Steroids and triterpenes: The extracts were dried and rediluted in 2 mL of chloroform that was separated in 2 tubes for the Salkowski test and the Liberman-Burchard reaction. For this last reaction, green coloration was taken as the presence of steroids, while a violet coloration indicated triterpenes. Reddish brown coloration of interface indicated the presence of Terpenes for Salkowski (Tiwari et al. 2011).
Tannins: The extracts were dried and re-dissolved with distilled water (3 mL) and 2 drops of ferric chloride were added. The blue coloration was taken as positive for hydrolysable tannins and green coloration for condensed tannins (Tiwari et al. 2011).
Quantification of flavonoids: An initial calibration curve was done with quercetin. For each of the Capsicum species, a mixture of 0.5 mL of the ethanolic extract (0.1 % v/v) with 0.5 mL of a solution of aluminum trichloride (2 %) was prepared. The absorption was read after 40 minutes at 420 nm in a UVVis spectrophotometer (Hitachi UV-Vis model 150-20). The total concentration of flavonoid was expressed as quercetin equivalents (mg of quercetin per g of plant extract), based on the calibration curve. All determinations were carried out in triplicates (Woisky and Salatino 1998).
Quantification of total phenols: The Folin-Ciocalteu colorimetric method was carried as reported in the literature (Singleton et al. 1965). For each of the Capsicum species 50 μL of the ethanolic extract was mixed to 125 μL of the Folin reagent, and 400 μL of sodium carbonate 7.1 % (w/w), adding distilled water up to 1000 μL. The reading was done at 760 nm using the spectrophotometer and a comparison was established with the standard curve using gallic acid as the phenolic standard. Each Capsicum extract was analyzed in triplicated and results were expressed as mg of equivalent of gallic acid per grams of extract.
Detection of compounds by gas chromatography coupled to mass spectrometry (GCMS): Two samples of each Capsicum species were prepared, one was the resulting fraction after the initial fractionation and partition procedure described above (1), and nitrogen containing compound sample (2). This later sample was prepared from 100 mg of each ethanolic extract that was dissolved in 3 mL of H2S04 (2 % w/v). This was re-extracted with diethilic ether and NaOH (20 % pH 9-10) was added and extracted again with ethyl acetate and dried. The resultant fraction (10 mg) was diluted in 5 mL of pyridine and N,O-bis (trimethylsilyl) trifluoroacetamide with 1 % of trimethylchlorodesilane (BSTFA+TMCS, Supelco), as derivatizing agents (Schummer et al. 2009). The mixture was heated for 30 min at 100 ºC. Furthermore, 5 μL of the samples (1) and (2), of C. baccatum and C. frutescens were injected in a gas chromatographer (Agilent 6890), coupled with a mass spectrometer (Agilent 5973), with a capillary column (Agilent 19091J-413, 30.0 m x 320.0 μm x 0.25 μm) using helium gas grade 5 (AGA Fano S.A., UAP 99.999 %) at a flux of 1 mL/ min (lineal velocity 37 cm/s). The injection was at split-less mode with a maximum temperature of 350 °C. The run was at SCAN mode with a waiting time of 6 min for the solvent, and an interval of masses between m/z 30-800. The chromatograms were analyzed with AMDIS software (Automated Mass Spectral Deconvolution and Identification System) (Augusto et al. 2000), and the spectral database NIST 98 (2001).
Results
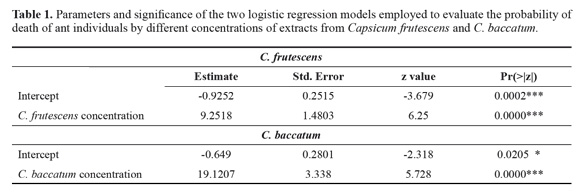
Insecticidal activity. The two species of Capsicum showed a positive and significant connection betwe en plant extract concentrations and the probability of death of ant individuals (Table 1; Fig. 1). The total residual deviance of the model was 0.98 for C. frutescens and 56.2 for C. baccatum.
However, the concentration of plant extract of C. baccatum needed to kill a predefined proportion of ant individuals was significant lower than that of C. frutescens (Table 2).
Antifungal activity. According to the ANOVA (Fig. 2), both species presented significant differences in their activity when compared to the control samples. In C. frutescens the antifungal activity was increased proportionally to the concentration, while for C. baccatum there were no difference between 0.1 and 0.25 % (w/v). Only at a concentration of 0.5 % w/v the activity was significantly different. Additionally, significant differences between C. baccatum and C. frutescens were found, in each of the concentrations evaluated (F = 141.4; df = 152; P < 0.001) (Fig. 2). There were no significant differences between both positive controls (with cellulose added and without).
Phytochemical analysis. In the qualitative colorimetric tests, cardiotonic glycosides, coumarins, steroids and triterpenes, alkaloids, and phenols were detected for both Capsicum species (Table 3).
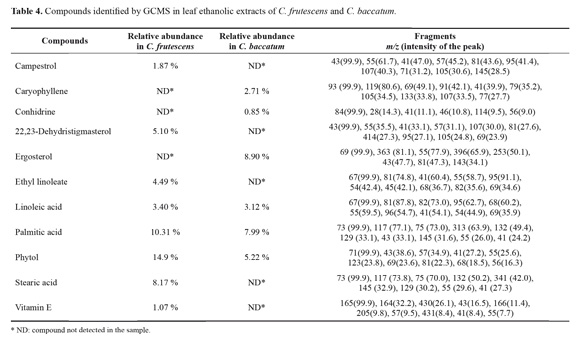
In the detection of compounds by GCMS, the chromatograms evidenced differences in the composition of volatile compounds between both species (Table 4). The most relevant finding in C. baccatum was the presence of caryophyllene and conhidrine, which were not detected in C. frutescens. In this last species, a high content of phytol, 22,23-dihydrostigmasterol, campesterol and some fatty acids ethyl esthers, were identified as the most abundant compounds (Table 4).
In the derivatized samples (nitrogen containing compounds) of C. frutescens, two characteristic molecular ions of m/z 224 and m/z 209 were evidenced, which were not detected in C. baccatum. These fragments are typical of TMSderivatized vanillylamine and a 4-hydroxy-3-methoxybenzyl fragment, respectively.
Discussion
In the search of methods to control leaf cutter ants, it is relevant to consider that the symbiotic relationship between ants of the genus Atta and the fungus L. gongylophorus, implies that the negative effect on the survival of one of the organisms involved will compromise the survival of the other (Seal 2006). However, as evidenced in the present study both species of Capsicum exhibited bioactivity against the ants and the fungi (Figs. 1 and 2), which is significant according to some authors that have pointed out that plants containing chemicals with both activities, are the most promising leads (Boulogne et al. 2012). In accordance to this argument, the study conducted by Diaz Napal and collaborators (2015) found a promising lead out of a screening of 89 native Argentinian plant species against Acromyrmex lundi and its symbiotic fungus. The best activity was found in Aristolochia argentina Griseb (Aristolochiaceae), from which the lactone, argentilactone was identified through bioassay-guided fractionation presenting antiforaging and antifungal activities. The authors suggest that the link between both activities is related to the type of chemical defenses in the plant developed as a protection mechanism, emphasizing in the importance of addressing at once various types of bioactivities (Diaz Napal 2015). In this sense, plant biomass-degrading enzymes that are in the fungal gongylidia play an important role in the biodegradation in fungal gardens (Aylward et al. 2015). Therefore some authors propose that secondary metabolites that inhibit fungal enzymatic activity could cause leaves to be rejected by forager ants (Nichols-Orians and Schultz 1990). The evolutionary relationship of plants and these insects has led to complicated interactions between these two groups, so plants that exert activity over the fungus and the ants could act efficiently in the integrated management of the leaf cutter ants by affecting both organisms simultaneously.
Together with enthomopathogenic microorganisms, plants are the most widely studied options in the leaf cutter ant control (Valderrama-Eslava et al. 2009; Boulogne et al. 2012, Montoya-Lerma et al. 2012, Diaz Napal et al. 2015). The Solanaceae is among the top five plant families with reported insecticidal activity and in the seventh place as an antifungal (Boulogne et al. 2012). Numerous species within this family have ecological importance because the production of compounds that affects insects belonging to most orders (Chowanski et al. 2016). In this family, phenolic compounds such as eugenol and cinnamaldehyde have been reported in Capsicum species and Lycopersicon esculentum Mill., while the monoterpene pulegone and the alkaloids tomatine and solamargine have been found in Capsicum and Solanum species, as compounds with insecticidal and antifungal properties (Güntner et al. 2000, Boulogne et al. 2012). Overall in the literature, it was established that the most cited compounds for the control of the leaf cutter ant were terpenoids, alkaloids, and phenols (Boulogne et al. 2012). In accordance, these nuclei were detected in both species of Capsicum in the phytochemical screening (Table 3), but differences in the presence of specific compounds were shown in the analysis through GCMS (Table 4). This particular chemical composition is reflected in the bioactivity, while C baccatum was 2.2 times more toxic than C. frutescens in the assays against A. cephalotes (Fig. 1), this later species exhibited a total inhibition of the fungal growth at the highest concentration (Fig. 2).
In terms of the three major groups of compounds reported for its antifungal and insecticidal activity, for terpenoids the most relevant finding was the presence of caryophyllene in C. baccatum (Table 4). These byciclic sesquiterpenes have been recognized contributing to the general resistance of plants affecting a wide range of fungi (Bakkali et al. 2008). Caryophyllene oxide was reported as a defensive compound in leaves of Hymenaea courbaril L. (Fabaceae) against ants of the genus Atta (Hubbell et al. 1983). Additionally, Howard et al. (1989) demonstrated that caryophyllene oxide presented adverse effects against the leaf cutter ant and inhibited completely its symbiotic fungus. Moreover, caryophyllenes have been recognized as one of most promising type of compounds in the control of the leaf cutter ants (Boulogne et al. 2012). A related group to triterpenes is sterols, which were identified in both Capsicum species (Table 4), and have shown toxicity for L. gongylophorus (Marsaro et al. 2004). Otherwise, alkaloids as the second most cited group of compounds in the control of leaf cutter ants, are frequently reported in members of the Solanaceae family, having a defensive role against fungi and insects (Boulogne et al. 2012, Chowanski et al. 2016), In particular, the piperidinic alkaloid conhidrine was detected for C. baccatum by GCMS (Table 4), which has been known for its activity as antibiotic, insecticidal, and antifungal (Gregorí- Valdés 2005). Other nitrogen containing compounds well known in Capsicum species are capsaicinoids. Capsaicin (8-methyl-N-vanillyl-6-nonenamide) has been reported as an antifungal (Ozcelik et al. 2011), and has shown activity against the crop pests Myzus persicae (Sulz) and Leptinotarsa decemlineata Say (Chowanski et al. 2016). In this way in the GCMS analysis, a molecular ion typical for TMS-derivatized vanillylamide at m/z 224 and a fragment of m/z 209 characteristic of 4-hydroxy-3-methoxybenzyl were detected in C. frutescens, as the basic precursors for capsaicin (Keum et al. 2012). Generally capsaicinoids are produced in the fruits, but some authors explain its translocation to leaves and twigs to accomplish a protective role for the plant (Broderick and Cooke 2009). Finally, the other relevant group of compounds detected quantitatively for both plant species was phenols (Table 3). This type of metabolites has been well recognized in chemical signaling. It has been discussed that the acidity of the sap is related to the presence of phenolic compounds, which are fungal inhibitors, as well (Davidson and Fisher 1991; Magalhães et al. 2008). Overall, a total of twenty chemical classes of secondary metabolites have been reported in the leaf cutter ant control (Boulogne et al. 2012). It is noticeable the specific advantages of plants containing compounds such as terpenoids and phenols, which are known to have strong chemical defensive activity against insects, bacteria and fungi (Karamanoli et al. 2000).
The results obtained in this study carried out as in vitro studies, are considered a first step in the use of Capsicum leaf extracts in the cutter ant control. The bioactivity found for C. baccatum and C. frutescens could be considered for the approach proposed by some authors of preparing a mixture of plant extracts (Montoya-Lerma et al. 2012). Additionally this supports the traditional practice of some communities in the Amazon that use a mixture of Capsicum species to control leaf cutter ants (Personal communication with Liced Agudelo). Furthermore, the use of plant extracts has to be supported by standardization studies since secondary metabolites in plants can increase or be produced de novo as a response to pest invasion (Miresmailli and Isman 2014). In this way by identifying the compounds responsible for the bioactivity and proving its presence in the extract is a step that has to be complemented by the in situ activity. Some authors point out that standardized plant extracts with a mixture of active phytochemicals should reduce the rate of evolution of conventional resistance compared with the selection pressure exerted by single pure toxin (Della Lucia et al. 2014). Plant extracts are a complex chemical mix, hence insects are affected by many different compounds that usually have a broad physiological activity, which reduces the probability of developing resistance. In this way, because the leaf cutter ant management is still very complex, research in plant derived products and follow-up studies in the field conditions, should be prioritized in the search of promising alternatives.
Conclusions
Both plant extracts exhibited a combination of insecticidal and antifungal activity, being C. baccatum the best lead as an insecticidal while, C. frutescens presented a better antifungal activity at high concentrations. In the qualitative phytochemical tests the same type of compounds were detected in both species of Capsicum. Chemically related compounds have similar actions, which supports the promising bioactivity obtained for both Capsicum species. Further studies in the isolation and identification of compounds, could support the standardization of these plant extracts.
Acknowledgements
Authors acknowledge Professors Álvaro Duque for his advice in the statistical analysis, and Jair Gaviria and the Instrumental Analysis Laboratory of the Universidad Nacional de Colombia-Sede Medellín, for the acquisition of spectral data. We also acknowledge to the "Departamento Administrativo de Ciencia, Tecnología e Inovación, COLCIENCIAS" for the financial support under the grant 495-2009. LC. Salazar in thankful for the financial support given by the program "Jovenes Investigadores" COLCIENCIAS 566. Authors acknowledge the reviewers for their input in improving the manuscript.
Literature cited
ABBOT, W. A. 1987. Classic paper: Abbot's formula. A method of computing the effectiveness of an insecticide. Journal of the American Mosquito Control Association 3: 302-303. [ Links ]
Agudelo, L. 2007. Plantas utilizadas en el manejo de la hormiga "propia arriera" (Atta sexdens F. Smith) en las chagras indigenas Ticuna (Sector sur PNN Amacayacu, Amazonas, Colombia). Undergraduate final report in Biology. Instituto de Biologia, Universidad de Antioquia. Medellin, Colombia. 64 p. [ Links ]
Aubad, P. 2010. Plantas usadas por las comunidades indígenas Ticuna del PNN Amacayacu para el control de la hormiga cortadora: Evaluación Biológica y búsqueda de metabolitos secundarios. Master Thesis. Escuela de Química. Universidad Nacional de Colombia. Medellín, Colombia. 125 p. [ Links ]
Augusto , F.; Valente, A. L. P.; DOS Santos TADA, E.; Rivellino, S. R. 2000. Screening of Brazilian fruit aromas using solid-phase microextraction- gas chromatography - mass spectrometry. Journal of Chromatography A 873: 117-127. [ Links ]
Aylward , F. O.; Khadempour, L.; tremmel, D. M.; mcdonald, B. R.; nicora, c. d.; wu, s.; moore, r. j.; orton , d. j.; monroe, m. e.; piehowski, p. d.; purvine , s. o.; smith, r. d.; lipton , m. s.; burnumjohnson, k. e.; currie, c. r. 2015. Enrichment and broad representation of plant biomass-degrading enzymes in the specialized hyphal swellings of Leucoagaricus gongylophorus, the fungal symbiont of leaf-cutter ants. PLoS One 10: 1-12. [ Links ]
Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. 2008. Biological effects of essential oils - a review. Food and Chemical Toxicology 46: 446-475. [ Links ]
Boulogne, I.; Petit, P.; Ozier-Lafontaine , J.; Desfontaines , L.; Loranger-Merciris, G. 2012. Insecticidal and antifungal chemicals produced by plants: a review. Environmental Chemistry Letters 10: 325-347. [ Links ]
Broderick, C. E.; Cooke, P. H. 2009. Fruit composition, tissues, and localization of antioxidants and capsaicinoids in Capsicum peppers by fluorescence microscopy. Acta Horticulturae 841: 85-90. [ Links ]
Bueno, F. C.; Godoy, M. P.; Leite, A. C.; Bueno, O. C.; Pagnocca, F. C.; Fernandes, J. B.; Hebling, M. J. A.; Bacci, M. Jr.; Vieira, P. C.; Silva , M. F. G. F. 2005. Toxicity of Cedrela fissilis to Atta sexdens rubropicola (Hymenoptera: Formicidae) and its symbiotic fungus. Sociobiology 45: 389- 399. [ Links ]
BUENO, O. C.; Morini, M. S. C.; Pagnocca, F. C.; Hebling, M. J. A.; Silva , O. A. 1997. Sobrevivéncia de operárias de Atta sexdens rubropilosa Forel (Hymenoptera, Formicidae) isoladas do formigueiro e alimentadas com dieta artificial. Anais da Sociedade Entomológica do Brasil 26: 107-113. [ Links ]
Chaves , M. C. 2006. Evaluación preliminar del compostaje "arrieron" para el control de la hormiga Atta cephalotes L. en Jamundi, Valle. Boletin del Museo Entomológico de la Universidad del Valle 7(1): 10-21. [ Links ]
Chowanski , S.; Adamski, Z.; Marciniak, P.; Rosinski, G.; Büyükgüzel, E.; Büyükgüzel, K.; Falabella, P.; Scrano, L.; Ventrella, E.; Lelario, F.; Bufo, S. A. 2016. A Review of bioinsecticidal activity of Solanaceae alkaloids. Toxins 8: 1-28. [ Links ]
Crawley , M. J. 2012. The R Book. 2nd Edition. John Wiley & Sons Ltd, Chichester, England. 949 p. [ Links ]
Davidson , D. E.; Fisher, B. L. 1991. Symbiosis of ants with Cecropia as a function of light regime. pp. 289-309. In: Huxley, C. R.; Cutler, D. F. (Eds.). Ant-plant interactions. Oxford University Press, Oxford, UK. 309 p. [ Links ]
de Britto , J. S.; Forti , L. C.; de Oliveira, M. A.; Zanetti, R.; Wilcken, C. F.; Zanuncio, J. C.; LOECK, A. E.; CALDATO, N; NAGAMOTO, N. S.; LEMES P. G.; da Silva Camargo, R. 2016. Use of alternatives to PFOS, its salts and PFOSF for the control of leaf-cutting ants Atta and Acromyrmex. International Journal of Research in Environmental Studies 3: 11-92. [ Links ]
Della Lucia, T. M. C. 2003. Hormigas de importancia económica en la región Neotropical. pp. 337-349. En: Fernández, F. (Ed.). Introducción a las hormigas de la región Neotropical. Instituto de Investigación de Recursos Biológicos Alexander von Humboldt, Bogotá, Colombia. 398 p. [ Links ]
Della Lucia, M. C.; Gandra, L. C.; Guedes, R. N. C. 2014. Managing leaf-cutting ants: peculiarities, trends and challenges. Pest Management Science 70: 14-23. [ Links ]
del Toro, I.; William, M. V.; Mackay , P.; Rojas, P.; Zapata -Mata , R. 2009. Hormigas (Hymenoptera: Formicidae) de Tabasco: explorando la diversidad de la mirmecofauna en las selvas tropicales de baja altitud. Dugesiana 16: 1-14. [ Links ]
DIAZ NAPAL, G. N.; BUFFA, L. M.; NOLLI, L.C.; DEFAGO, M. T.; VALLADARES, G. R.; CARPINELLA, M. C.; RUIZ, G.; PALACIOS, S.M. 2015. Screening of native plants from central Argentina against the leaf-cutting ant Acromyrmex lundi (Guerin) and its symbiotic fungus. Industrial Crops and Products 76: 275-280. [ Links ]
DOHM, C.; LEAL, I. R.; TABARELLI, M.; MEYER, S. T.; WIRTH, R. 2011. Leaf-cutting ants proliferate in the Amazon: an expected response to forest edge? Journal of Tropical Ecology 27: 645-649. [ Links ]
DOS SANTOS, A.; ZANETTI, R.; DOS SANTOS, J. C.; BIAGIOTTI, G.; EVANGELISTA, A. L.; SERRAO, J. E.; ZANUNCIO, J. C. 2016. Persistence of fipronil residues in Eucalyptus seedlings and its concentration in the insecticide solution after treatment in the nursery. Environmental Monitoring and Assessment 188: 314. [ Links ]
Fernandes, J. B.; David , V.; Facchini, P. H.; da Silva , M. F. das G. F.; Rodrigues, E.; Vieira, P. C. 2002. Extrações de óleos de sementes de citros e suas atividades sobre a formiga cortadeira Atta sexdens e seu fungo simbionte. Química Nova 25 (6B): 1091-1095. [ Links ]
FernÁndez, F.; Castro-Huertas , V.; Serna, F. 2015. Hormigas cortadoras de hojas de Colombia: Acromyrmex y Atta (Hymenoptera: Formicidae). Fauna de Colombia-Monografía No. 5. Universidad Nacional de Colombia. 350 p. [ Links ]
Forti , L. C.; Pretto , D. R.; Nagamoto , N. S.; Padovani , C. R.; Camargo, R. S.; Andrade, A. P. P. 2007. Dispersal of the delayed action insecticide sulfluramid in colonies of the leaf-cutting ant Atta sexdens rubropilosa (Hymenoptera: Formicidae). Sociobiology 50: 1-15. [ Links ]
Giraldo, C. 2008. Microbiota asociada a la casta obrera de la hormiga arriera Atta cephalotes (Hymenoptera: Myrmicinae) y su posible relación con la división de tareas. M. Sc. Thesis. Departamento de Biología. Universidad del Valle. Cali, Colombia. 66 p. [ Links ]Gregorí-Valdés, B. S. 2005. Estructura y actividad de los antifúngicos. Revista Cubana de Farmacia 39: 1-15. [ Links ]
Güntner, C.; Vazquez, A.; Gonzalez, G.; Usubillaga, A.; Ferreira, F.; Moyna, P. 2000. Effect of Solanum glycoalkaloids on potato aphid, Macrosiphum euphorbiae: part II. Journal of Chemical Ecology 26: 1113-1121. [ Links ]
Herrera, M.; Valenciaga, N. 2011. Peculiarities of leafcutter ants (Attini: Acromyrmex and Atta) that make difficult their control. Cuban Journal of Agricultural Science 45: 217. [ Links ]
HOWARD, J. J.; CAZIN, J. J.; WIEMER, D. F. 1988. Toxicity of terpenoid deterrents to the leaf cutting ant Atta cephalotes and its mutualistic fungus. Journal of Chemical Ecology 14: 59-69. [ Links ]
Howard , J.; Green, T.; Wiemer, D. 1989. Comparative deterrency of two terpenoids to two genera of attine ants. Journal of Chemical Ecology 15: 2279-2288. [ Links ]
Hubbell, S.; Weimer, D.; Adejare, A. 1983. An antifungal terpenoid defends a neotropical tree (Hymenaea) against attack by fungus-growing ants (Atta). Oecologia 60: 321-327. [ Links ]
Kavit , M.; Patel , B. N.; Jain, B. K. 2013. Phytochemical analysis of leaf extract of Phyllanthus fraternus. Research Journal of Recent Sciences 2: 12-15. [ Links ]
Karamanoli, K.; Vokou, D.; Menkissoglu, U.; Constantinidou , H.-I. 2000. Bacterial colonization of phyllosphere of Mediterranean aromatic plants. Journal of Chemical Ecology 26: 2035-2048. [ Links ]
Keum, S. Y.; Park, H. W.; Song, H. H.; Kim, B. D.; Kang, B. C.; Kim, J. H. 2012. Metabolite analysis of long chain branched fatty acids and capsaicin biosynthesis in Capsicum annum placenta. Journal of Korean Society of Applied Biological Chemistry 55: 189-195. [ Links ]
Lee, M. R. 2006. The Solanaceae: Foods and poisons. Journal of the Royal College of Physicians of Edinburgh 36: 162-169. [ Links ]
LOCK, O. 1988. Investigación fitoquímica: métodos en el estudio de productos naturales. Fondo Editorial Pontificia Universidad Católica del Perú, Lima, Peru. 213 p. [ Links ]
Loeck, A. E.; GusmÃo, L. G. 1998. Controle de Acromyrmex heyeri Forel, 1998 e Acromyrmex ambiguus Emery, 1887 (Hymenoptera: Formicidae) com fluramim na localidade de Pelotas, RS. Revista Brasileira de Agrociencia 4: 59-63. [ Links ]
López, E.; Orduz, S. 2003. Metarhizium anisopliae and Trichoderma viride for control of nests of the fungus-growing ant, Atta cephalotes. Biological Control 27: 194-200. [ Links ]
Magalhães, L. M.; Segundo, M. A.; Reis, S.; Lima, J. L. 2008. Methodological aspects about in vitro evaluation of antioxidant properties. Analytica Chimica Acta 613: 1-19. [ Links ]
Marsaro, A. L. Jr.; Souza, R. C.; Della Lucia, T. M. C.; Fernandes, J. B.; Silva M. F. G. F.; Vieira; P. C. 2004. Behavioral changes in workers of the leaf-cutting ant Atta sexdens rubropilosa induced by chemical components of Eucalyptus maculata leaves. Journal of Chemical Ecology 30: 1771-1780. [ Links ]
Maya , B. S. M. 2002. Influencia de factores edáficos y de los componentes del patosistema Fusarium oxysporum sp. ciceris / Cicer arietinum en el desarrollo de la fusariosis vascular del garbanzo. Ph. D. Thesis. Universidad de Cordoba. Cordoba, Spain. 283 p. [ Links ]
Miresmailli, S.; Isman, M. B. 2014. Botanical insecticides inspired by plant-herbivore chemical interactions. Trends in Plant Science 19: 29-35. [ Links ]
MOntoya -lerma, j.; giraldo-echeverri, c.; armbrecht, i.; farji -brener, a.; calle, z. 2012. Leafcutting ants revisited: Towards rational management and control. International Journal of Pest Management 58: 225- 247. [ Links ]
NICHOLS-ORIANS, C. M.; SCHULTZ, J. C. 1990. Interactions among leaf toughness, chemistry, and harvesting by attine ants. Ecological Entomology 15: 311-320. [ Links ]
Ortiz , A. 1998. Selección y evaluación de una cepa de Trichoderma o Gliocladium para el control de Atta cephalotes en condiciones de laboratorio. Facultad de Ciencias. Universidad Nacional de Colombia. Medellín, Colombia. 122 p. [ Links ]
ORTIZ, A.; GUZMÁN, G. E. 2007. Las hormigas cortadoras de hojas del Departamento de Antioquia. 1st ed. Universidad de Antioquia, Universidad Nacional de Colombia. Medellín, Colombia. 111 p. [ Links ]
OLIVEIRA, M. F. S. S. 2006. Controle de formigas cortadeiras (Hymenoptera: Formicidae) com productos naturais. Instituto de Biociencias. Ph. D. Thesis. Universidade Estadual Julio de Mesquita Filho: Rio Claro, Brazil. 119 p. [ Links ]
Ozcelik, B.; Kartal , M.; Orhan, I. 2011. Cytotoxicity, antiviral and antimicrobial activities of alkaloids, flavonoids, and phenolic acids. Pharmaceutical Biology 49: 396-402. [ Links ]
Pagnocca, F.; Ribeiro, S.; Torkomian, V.; Hebling, M.; Bueno, O.; da Silva , O.; Fernandes, J.; Vieira, P.; da Silva , M.; Ferreira, A. 1996. Toxicity of lignans to symbiotic fungus of leaf-cutting ants. Journal of Chemical Ecology 22: 1325-1330. [ Links ]
Paoletti, M. G.; Pimentel, D. 2000. Environmental risks of pesticides versus genetic engineering for agricultural pest control. Journal of Agricultural and Environmental Ethics 12: 279-303. [ Links ]
Parekh, J.; Chanda, S. 2007. Antibacterial phytochemical studies on twelve Indian medicinal plants. African Journal of Biomedical Research 10: 175-181. [ Links ]
Peñaflor, V. M. F. G.; Almeida, R. N. A.; Simote, S. Y.; Odair, E. Y.; Bueno, C.; Püntener W. 1981 Manual for field trials in plant protection. Second edition. Agricultural Division, Ciba-Geigy Limited. [ Links ]
R Development Core Team. 2012. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. ISBN 3-900051-07-0. Disponible en: http://www.R-project.org/ [Review date: June 2016] [ Links ].
Rauh, V. A.; Perera, F. P.; Horton , M. K.; Whyatt , R. M.; Bansal, R.; Hao, X.; Liu, J.; Barr, D. B.; Slotkin, T. A.; Peterson, B. S. 2012. Brain anomalies in children exposed prenatally to a common organophosphate pesticide. Proceedings of the National Academy of Sciences 109: 7871- 7876. [ Links ]
Schummer, C.; Delhomme, O.; Appenzeller, B. M.; Wennig, R.; Millet, M. 2009. Comparison of MTBSTFA and BSTFA in derivatization reactions of polar compounds prior to GC/MS analysis. Talanta 77: 1473-1482. [ Links ]
Seal, J. N. 2006. Self-organization and the superorganism: functional ecology of the obligate mutualism between a fungus gardening ant and its symbiotic fungus. PhD Thesis. Florida State University. Florida, USA. 89 p. [ Links ]
Serna, F. J.; Correa, J. A. 2003. Extractos de hojas de tomate Lycopersicon esculentum como fagoinhibidores de Atta cephalotes. Revista Agronomia Colombiana 21: 142-153. [ Links ]
Singleton , V. L.; Rossi, J. A. 1965. Colorimetry of total phenolics with phosphomolybdic–phosphotungstic acid reagents. American Journal of Enology and Viticulture 16: 144- 158. [ Links ]
Tiwari , P.; Kumar, B.; Kaur, M.; Kaur, G.; Kaur, H. 2011. Phytochemical screening and extraction: a review. Internationale Pharmaceutica Sciencia 1: 98-106. [ Links ]
Valderrama-Eslava , E. I.; Montoya -Lerma, J.; Giraldo, C. 2009. Enforced herbivory on Canavalia ensiformis and Tithonia diversifolia and its effects on leafcutting ants, Atta cephalotes. Journal of Applied Entomology 133: 689-694. [ Links ]
Woisky, R. G.; Salatino , A. 1998. Analysis of propolis: some parameters and procedures for chemical quality control. Journal of Apicultural Research 37: 99-105. [ Links ]
YUSUF, a. z.; Zakir, A.; Shemau, Z.; Abdullahi, M.; Halima, S. A. 2014. Phytochemical analysis of the methanol leaves extract of Paullinia pinnata Linn. Journal of Pharmacognosy and Phytotherapy 6: 10-16. [ Links ]
Zavan , C. 2005. Identificação de inibidores de pectinase fúngica para o controle de formigas cortadeiras. Tesis de Maestría en Microbiología Aplicada. Universidad Estadual Paulista. Instituto de Biociencias. Rio Claro, Brazil. 96 p. [ Links ]
Received: 11-Sep-2015
Accepted: 26-May-2016