Introduction
Raoiella indica (Hirst, 1924) (Trombidiformes: Tenuipalpidae), the red palm mite, was originally described from India attacking coconut, Cocos nucifera L., 1753 (Hirst 1924). Other host plants recorded in previous studies were Phoenix dactylifera L., 1753, Dictyosperma album (Bory, 1804) (Womersley 1943; Moutia 1958) and Areca catechu L., 1753 (Daniel 1979). These hosts belong to Arecales: Arecaceae.
The mite was widespread throughout countries bordering the Indian Ocean, with its western limit in Egypt and Israel (Gerson et al. 1983). It was detected for the first time in the New World in Martinique in 2004 (Flechtmann and Etienne 2004). It then spread to other Caribbean islands and later to continental America. Currently, it is found in Barbados, Brazil, Colombia, Cuba, Dominica, Dominican Republic, Grenada, Guadeloupe, Haiti, Jamaica, Martinique, Mexico, Puerto Rico, Saint Lucia, Saint Vincent and the Grenadines, Trinidad and Tobago, United States of America (Florida and Virgin Islands) and Venezuela (CABI 2016).
After its arrival on the American continent it has broadened the host range, but the preferred hosts belong to Musaceae and Arecaceae (Flores et al. 2010). Carrillo et al. (2012a) listed 91 species of plant hosts; this list continues to grow as the mite settles in new territories (González and Ramos 2010; Gondim et al. 2012; Gómez-Moya et al. 2017).
R. indica and all other tenuipalpid mites are phytophagous. However, this species differs from other con-familial species by feeding on stomatal cells (Beard et al. 2012). It reproduces sexually and also by arrhenotokous parthenogenesis (Helle et al. 1980). Its life cycle includes egg, larval, protonymphal, deutonymphal and adult stages of both sexes. At the end of each stage, it passes through a quiescent period when specimens extend their legs and neither move nor feed. Female predominance varies seasonally, 92 % during spring and 70 % during autumn (Moutia 1958).
The injuries inflicted by this mite on its host plants are severe, and it is considered a major invasive pest (Mendonça et al. 2005). Infestation levels are strongly seasonal, increasing in the dry and hot season, and spontaneously decreasing at the onset of the rainy season. It is less important in temperate or cold regions, being mostly a problem in tropical countries (Peña et al. 2010).
This study was conducted to determine fecundity life table parameters of R. indica on coconut leaflets, under different temperature and humidity conditions (Birch 1948).
Materials and methods
Rearing R. indica
Coconut plants purchased in a R. indica-free area (Puente Nacional, Veracruz, Mexico, 2015, located at 19º19’38”N, 96º28’53”W, 104 masl) were carefully examined to verify they were not infested by this mite; any insect or mite present was manually eliminated. Using a fine brush, live R. indica specimens were transferred to those plants and kept in a growing chamber at 26 °C, photoperiod 12:12 h (light: dark), to establish colonies; mites were taken from the colonies as needed. All observations were carried out in Colegio de Postgraduados, Texcoco, Mexico, located at 19º27’46”N, 98º54’15”W, 2,246 masl.
Life tables at different temperatures
Four cohorts of almost uniform age were formed with 50 R. indica eggs each. To this end, leaflets highly infested by this mite were selected, removing all eggs with a fine brush. The leaflets were examined 24 h later, and all eggs laid during this period were assumed to be 12 h old. Leaflet sections (8 x 5 cm) were cut from mite-free coconut plants, removing all mites or insects under a stereomicroscope, and leaving a single egg on the abaxial surface of each section. The base of each section was covered with a cotton pad, and between the leaflet and the cotton pad an adhesive barrier line (Tanglefoot ™) was drawn. Two hundred and twenty arenas were prepared and placed in four groups of 50 (cohorts) in plastic pans. The remaining 20 were used to replace mites that died in early stages, likely because of manipulation.
Each of these egg cohorts was kept in an incubator (Thermo Scientific mod. TFFU2065FWA) previously calibrated to 22.5, 25, 27.5 or 30 ± 1 °C, with a 12:12 h (light: dark) photoperiod. The cotton pads were kept moist to prevent leaflet sections from wilting, and additional pans with water were put in the incubators until relative humidity was 40-50 %. When R. indica eggs hatched, each larva was transferred with a fine brush to a new arena where the leaflet section was placed abaxial surface down, at a 45° angle from horizontal. Arenas were observed once a day to follow development and survival of individual mites. Mites were transferred to new arenas approximately every 15 days when leaflets began to wither. Given that the gender of R. indica is easily recognizable only when they attain adulthood, survival curves for each cohort were drawn combining specimens of both genera.
Fecundity life tables at different temperatures
To construct fecundity tables for each cohort of R. indica, females reaching adulthood were registered. Two male mites about 24 h old were placed close to each young female for mating and left on the arena until their death. The process of rearing and selecting males is subsequently explained. The number of eggs laid by each female was recorded once a day. Counted eggs were removed from the arena so they would not be counted again.
According to Vera et al. (2002), the following parameters of population increase were estimated: generational time (Tg), net reproductive rate (Ro), finite rate of increase (λ) and intrinsic rate of increase (rm). Estimation of the above parameters for R. indica implied several problems: the gender of the mites was recognized only when they attained adulthood; the proportion of sexes is biased towards females, which constitute between 70 and 90 % of total specimens (Moutia 1958); many specimens died in a pre-adult stage, so their gender could not be determined. In order to estimate the proportion of females to males, the gender of the mites from each cohort was determined the first day all had attained adulthood. Based on this data and supported by data of Moutia (1958), for estimation of population increase parameters, it was assumed that 80 % of all specimens in a cohort were females; similarly, 80 % of total eggs laid by the females were assumed as daughters.
Fertility life tables at different levels of relative humidity
Fertility life tables were constructed for mites reared at 27.5 ± 1 °C (temperature at which the highest rm occurred) and relative humidity ranges of 30-40, 60-70 or 80-90 %. Mite cohorts were founded and handled as previously explained. To regulate relative humidity inside the incubators, temperature was first set at 27.5 °C and then an increasing number of water-filled pans were placed until humidity ranged between the pre-established limits. Relative humidity was monitored daily and water was added as needed.
Mating behavior
Fifteen, approximately 24 h, old adult males of R. indica were obtained to fertilize females in the fertility life tables. For their capture, highly infested coconut leaflets were examined to obtain quiescent male deutonymphs, which were recognized by the pointed tip of their opisthosoma. They were transferred to coconut leaflets and, about 24 h after they molted (became adults), two of them were transferred with a fine brush to an arena where there was one female.
To determine how many times a male can mate, the males were placed individually on arenas, and a quiescent female deutonymph was transferred to each one arena, close to the male. The deutonymphs were recognized as females by their round opisthosoma. Each arena was observed at hourly intervals and, once a female started the molting process, it was observed continuously to record if the male mated her. After mating, the female was removed and, the following day, a new quiescent female deutonymph was introduced. This procedure was repeated until the male mite died.
Data analysis
Survival curves of mite cohorts reared under different combinations of temperature and relative humidity were compared using the Logrank test (Vera et al. 2002). Using the overlapping intervals test (Vera and Sotres 1991), rm values and their confidence intervals were used to estimate projections of population increase and recognize differences among them. Development of individual mites was followed until they died. When the number of observed specimens was ≥ 3, numerical values were subjected to an ANOVA and Fisher’s least significant difference using SAS 9.0 (2002).
Results
Survival curves at different temperature and relative humidity
In all combinations of temperature and relative humidity, mortality of combined females and males occurred evenly along the entire life cycle (Figs. 1 and 2). The apparent zero mortality during the first days may have been due to difficulty in distinguishing a live egg from a dead one. In contrast, an increase in mortality occurred between days 6 and 20, when larvae and nymphs prevailed. Larvae and nymphs are soft and delicate, and could have died from manipulation. The above facts might lead to underestimating egg mortality, and to overestimating larval and nymph mortality.

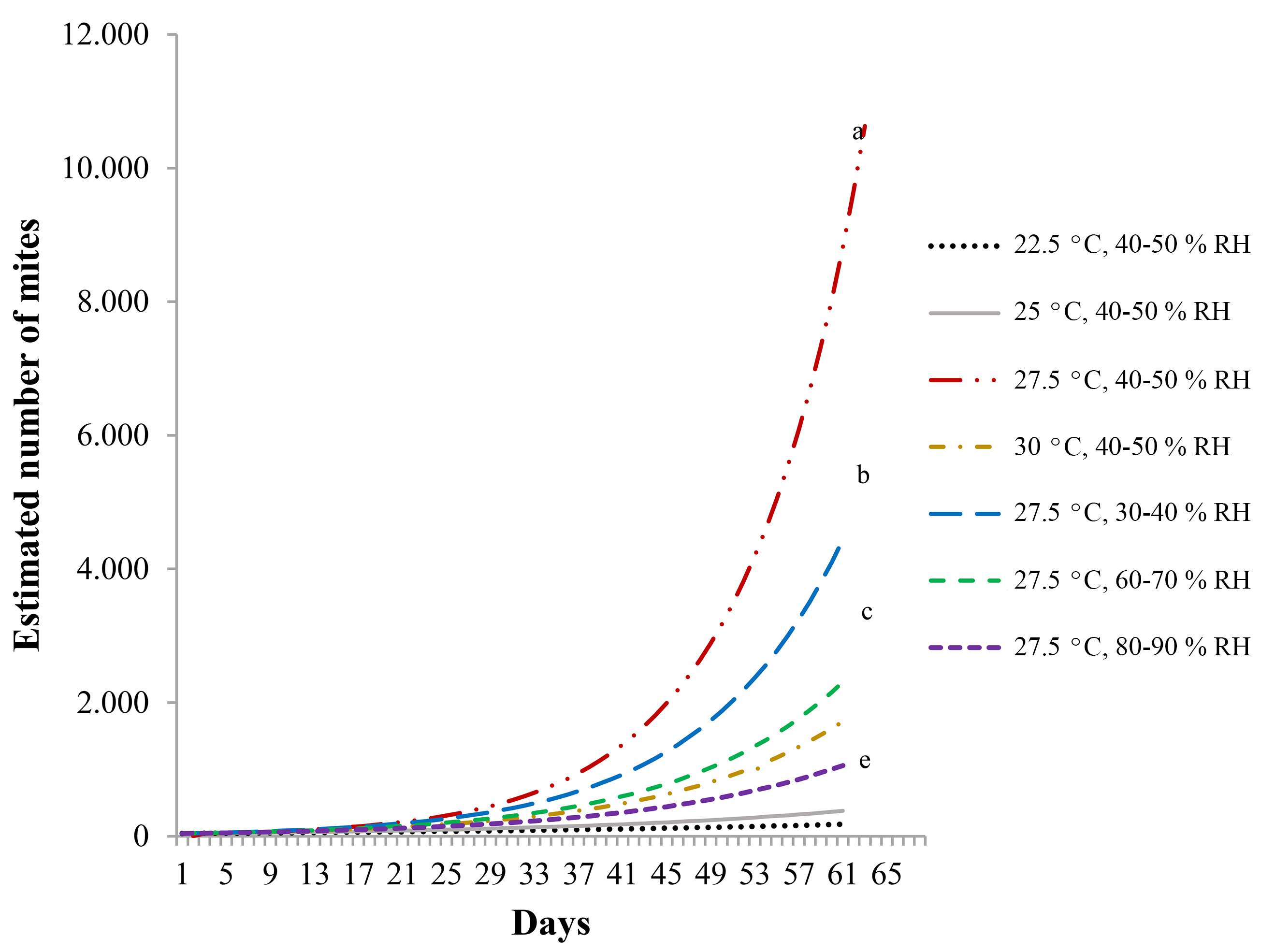
Figure 1 Survival curve of Raoiella indica reared at four temperatures, relative humidity 40-50 %. The day when eggs were laid is defined as day zero.

Figure 2 Survival curves of Raoiella indica reared in three ranges of relative humidity at 27.5 ± 1 °C. The day when eggs were laid is defined as day zero.
A clear inverse relation was found between temperature and mite lifespan. The survival curve of mites reared at 22.5 °C, 40-50 % RH, was significantly different from all the other curves (Logrank test, P = 0.05, Table 1). The longest life span of mites reared at that temperature was about 100 days. The survival curve of mites reared at 30 °C, 40-50 % RH, was significantly different from those of mites reared at 27.5 °C, with several combinations of relative humidity (Table 1). The longest life span of mites reared at 30 °C was 39 days (Fig. 1).
In contrast, when temperature was the same (27.5 °C) but relative humidity varied, all survival curves followed almost parallel courses and they were not significantly different (Logrank test, P = 0.05). None of the relative humidity ranges particularly affected the R. indica survival pattern (Fig. 2; Table 1).
Table 1 Values of χ2 for comparison of survival curves of Raoiella indica at different temperatures and ranges of relative humidity, Logrank test (Vera et al. 2002).
| 25 °C, 40-50 % RH | 27.5 °C, 40-50 % RH | 30 °C, 40-50 % RH | 27.5 °C, 30-40 % RH | 27.5 °C, 60-70 % RH | 27.5 °C, 80-90 % RH | |
|---|---|---|---|---|---|---|
| 22.5 °C, 40-50 % RH | 17.23* | 7.87* | 27.49* | 8.81* | 24.96* | 10.04* |
| 25 °C, 40-50 % RH | 0.55 | 2.75 | 51.36* | 1.87 | 0.0003 | |
| 27.5 °C, 40-50 % RH | 26.16* | 0.06 | 0.01 | 0.57 | ||
| 30 °C, 40-50 % RH | 26.16* | 0.0006 | 0.57 | |||
| 27.5 °C, 30-40 % RH | 0.06 | 0.44 | ||||
| 27.5 °C, 60-70 % RH | 0.18 |
* Significantly different, P = 0.05.
Effect of temperature and relative humidity on rate of R. indica development
The duration of each life stage, as well as of the complete immature phase, was inversely related to temperature for both sexes together. The shortest developmental time was observed at 30 °C (Tables 2 and 3). In contrast, there was no clear relation between levels of relative humidity (at 27.5 °C) and duration of life stages. Significant differences in duration of some life stages were observed, but there was no level of relative humidity that consistently resulted in longer or shorter life stages.
Table 2 Duration of life stages of Raoiella indica females reared at different temperatures and levels of relative humidity.
| Temp. °C/ % RH | n | Eg | Lv A | Lv Q | Pn A | Pn Q | Dn A | Dn Q | Time Eg-Ad | Longev | Preov | Ov | Postov |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 22.5/40-50 | 13 | 9.6 a* | 5.2 a | 3.6 a | 4.8 a | 3.3 a | 6.9 a | 4.4 a | 38.9 a | 30.9 a | 4.4 ab | 23.8 ab | 2.9 b |
| 25/40-50 | 11 | 7.4 b | 2.9 c | 3.0 b | 3.5 b | 2.4 b | 4.7 b | 3.0 b | 27.7 b | 32.0 a | 4.64 a | 26.4 a | 2.0 b |
| 27.5/40-50 | 33 | 6.7 c | 2.3 e | 1.9 d | 2.1 c | 1.6 d | 2.9 cd | 2.1 c | 20.7 de | 22.4 b | 3.1 dc | 17.1 bc | 2.2 b |
| 30/40-50 | 23 | 4.6 e | 2.0 e | 1.6 d | 2.1 c | 1.2 e | 2.2 cd | 1.5 d | 17.0 f | 12.8 c | 2.7 d | 9.6 c | 1.8 b |
| 27.5/30-40 | 23 | 5.6 d | 2.5 de | 2.0 c | 2.3 c | 2.0 bc | 2.6 cd | 1.9 d | 19.9 e | 28.8 ab | 3.3 bdc | 22.8 ab | 2.9 b |
| 27.5/60-70 | 29 | 6.9 bc | 2.9 cd | 2.3 bc | 2.5 c | 1.9 cd | 2.4 d | 2.1 c | 21.9 dc | 22.7 b | 3.0 dc | 17.2 bc | 2.6 b |
| 27.5/80-90 | 17 | 4.7 e | 3.7 b | 2.2 bc | 3.4 b | 1.8 cd | 3.0 c | 2.7 b | 22.4 | 28.2 ab | 4.1 abc | 19.7 ab | 4.6 a |
* Values in the same column and with the same letter are not significantly different (least significant difference, P = 0.05). n: number of replicates, Eg: egg, Lv: larva, Pn: protonymph, Dn; deutonymph, Ad: adult, Longev: longevity, Preov: preoviposition, Ov: oviposition, Postov: postoviposition, A: active, Q: quiescent.
Table 3 Duration of life stages of Raoiella indica males reared at different temperatures and levels of relative humidity.
| Temp. °C/ % RH | n | Eg | Lv A | Lv Q | Pn A | Pn Q | Dn A | Dn Q | Time Eg-Ad | Longev |
|---|---|---|---|---|---|---|---|---|---|---|
| 22.5/40-50 | 10 | 9.5 a* | 4.4 a | 3.5 a | 4.6 a | 3.1 | 5.0 a | 3.7 a | 34.8 a | 37.7 a |
| 25/40-50 | 2 | 8.5 - | 4.0 - | 3.5 - | 4.6 - | 3.1 - | 5.0 - | 3.7 - | 24.3 b | 37.7 - |
| 27.5/40-50 | 3 | 6.7 b | 2.0 c | 2.0 b | 2.7 ab | 1.7 b | 2.0 bc | 2.3 b | 20.3 bc | 26.0 ab |
| 30/40-50 | 4 | 8.2 c | 3.6 bc | 3.0 b | 4.0 b | 2.6 b | 4.0 bc | 3.2 b | 17.5 c | 33.8 c |
| 27.5/30-40 | 10 | 6.0 bc | 2.3 c | 1.4 b | 3.0 ab | 2.0 b | 1.6 c | 1.8 b | 19.1 c | 16.0 bc |
| 27.5/60-70 | 2 | 7.0 - | 3.0 - | 2.5 - | 2.0 - | 1.5 - | 2.0 - | 2.5 - | 21.5 bc | 7.5 - |
| 27.5/80-90 | 10 | 5.1 c | 3.4 b | 2.0 b | 3.1 ab | 1.9 b | 2.8 b | 2.1 b | 21.4 bc | 20.9 bc |
* Values in the same column with the same letter are not significantly different (least significant difference, P = 0.05). n: number of replicates, Eg: egg, Lv: larva, Pn: protonymph, Dn; deutonymph, Ad: adult, Longev: longevity, A: active, Q: quiescent.
Parameters of population increase
The proportion of females to total adult specimens was 0.793 ± 0.147 (standard deviation). Given that the cohorts were established with eggs of unknown gender, the differences in proportion of genders among cohorts cannot be attributed to rearing conditions.
Parameters of population increase for different combinations of temperature and relative humidity are shown in Table 4. In all cases, rm was positive, showing that no combination of temperature and humidity limited R. indica population increase. It was clear, however, that temperature was the main factor affecting the intrinsic rate of increase. The effect of temperature can also be appreciated in both generational time and indices of fecundity (λ and Ro). The highest rm value occurred when temperature was 27.5 °C and relative humidity 40-50 %. These conditions are thus the closest to optimal for development and population increase of R. indica.
With the test of overlapping intervals of Vera and Sotres (1991), projections of population increase were drawn (Fig. 3). The results confirm temperature as the main factor affecting R. indica population increase, with 27.5 °C as closest to the optimal. At 22.5 °C, population increase is very slow, suggesting that injuries to host plants R. indica may cause under those conditions would be reduced. There was also an inverse relation between relative humidity and population increase, where curves of population increase were significantly different.
Fecundity curves at different temperatures and levels of relative humidity
Of all eggs laid by R. indica females during their entire oviposition periods at different combinations of temperature and relative humidity, 80 % were assumed to be females (daughters) (Figs. 4 and 5). A clear association with temperature was evident (Fig. 4). Females reared at 30 °C concentrated oviposition between days 15 and 37, with the highest rate from day 20 to day 29. In contrast, when reared at 22.5 °C, females laid eggs between days 37 and 92, peaking towards the center of that lapse. At 25 and 27.5 °C, fecundity curves followed similar patterns, egg-laying taking place between days 30 and 70, although total oviposition was higher at 27.5 °C.
Even though mites reared at 30 °C started egg laying long before those at other temperatures, the number of descendants (daughters) was lower, a fact that resulted in a lower net reproductive rate (Ro = 4.2). Mites reared at 22.5 °C started laying eggs later and continued longer; however, their Ro was also 4.2. Temperature resulting in the highest Ro (11.92) and rm (0.0856) was 27.5 °C (Table 4).
In contrast, fecundity curves of mites reared at the same temperature but different levels of relative humidity started and ended at similar times and followed similar patterns, resulting in similar Ro values (Fig. 5). This shows that humidity alone at the tested levels did not have a significant effect on R. indica oviposition.
Table 4 Parameters of population increase of Raoiella indica at temperatures from 22.5 to 30 °C and relative humidity from 30-40 to 80-90 %.
| Rearing conditions | R o | T g | r m | λ |
|---|---|---|---|---|
| 22.5 °C, 40-50 % RH | 4.22 | 59.341 | 0.0242 | 1.0245 |
| 25 °C, 40-50 % RH | 5.38 | 46.617 | 0.0361 | 1.0367 |
| 27.5 °C, 40-50 % RH | 19 | 34.377 | 0.0856 | 1.0894 |
| 30 °C, 40-50 % RH | 4.2 | 23.362 | 0.0614 | 1.0633 |
| 27.5 °C, 30-40 % RH | 11.92 | 34.347 | 0.0721 | 1.0748 |
| 27.5 °C, 60-70 % RH | 8.84 | 35.323 | 0.0617 | 1.0636 |
| 27.5 °C, 80-90 % RH | 6.48 | 35.935 | 0.0546 | 1.0561 |
Mating behavior
When R. indica males were put in contact with female deutonymphs and once they reached adulthood, they were capable of mating with three to six of them (mean 4.56, n = 15) for their whole life. Mating took place almost immediately after the females molted (sometimes only partially). Whether a male mated more than once with the same female was not observed.
Discussion
The use of detached leaves, leaf discs or similar plant parts to prepare arenas, is a useful tool to follow development and reproduction of individual mites, mainly to construct life tables. However, some degree of artificiality is unavoidable, not only because there could be water stress that causes leaves or leaf discs to wilt after a variable number of days (Helle and Overmeer 1985), but because there is no competition (intra-or interspecific), there are no predators, temperature and humidity are kept almost constant, leaves are usually placed with the abaxial surface up, food is theoretically unlimited, etc. (Vera et al. 2002).
This could be particularly relevant for Raoiella spp., that feed by inserting their cheliceral stylets through plant stomata (Ochoa et al. 2011). The use of leaf pieces is expected to result in closure of stomata, a fact affecting the capacity of mites to feed, and then the life cycle and reproduction would be affected (Beard et al. 2012). Cocco and Hoy (2009) failed to settle R. indica on leaf discs of banana and plantain, likely because discs wilted and consequently stomata closed. However, they also failed to settle this mite on potted banana and plantain plants, so other factors could be involved beside closure of stomata, such as the age of leaves, as studied by Otero-Colina et al. (2016).
In contrast, R. indica can be reared on coconut leaf pieces, as shown by Cocco and Hoy (2009), González and Ramos (2010) and Vásquez et al. (2015). There is no information about how long after coconut leaves are cut their stomata close (different from its normal function); similarly, it has not been determined if closed stomata impede R. indica mite feeding. To prolong the useful life of leaf sections, in the present study, 8 x 5 cm sections were used, instead of smaller portions (18 x 45 mm) as used by Cocco and Hoy (2009); their base was covered with water soaked cotton pads and those sections were replaced when they started to lose a turgid aspect.
As a result, in our research mites could reach adulthood and reproduced; life span and fecundity were similar to those estimated for R. indica in other studies under conditions closest to what we found as the optimal ones (Moutia 1958; Nageshachandra and Channabasavanna 1984). As population parameters can be compared between different environmental conditions, provided mites are handled the same way, it is postulated that the obtained values really represent the effect of temperature and relative humidity on development and reproduction of R. indica, the aim of this study.
All survival curves observed in this study, regardless of the combination of temperature and relative humidity, were type III according to the classification presented by Vera et al. (2002). In this type of survival curve, mortality is evenly distributed over time. This was consistent throughout our study. Survival curves drawn for other tenuipalpid species are different from those we observed. In two life tables of a species identified as Brevipalpus phoenicis (Geiskes, 1936) (Kennedy et al. 1996; Teodoro and Reis 2006), almost all specimens died at relatively advanced life stages, with type I survival curves (Vera et al. 2002). In another study, Tenuipalpus heveae (Baker, 1945) (also Tenuipalpidae) was shown to have high larval mortality, but most of the remaining specimens remained alive until adulthood (Feres et al. 2010). Thus, their survival curve was similar to type IV (Vera et al. 2002). The survival curves observed in the present study show that a lower proportion of R. indica survives to adulthood, and this was expected to have an effect on the parameters of population increase.
Consistently, the length of R. indica lifespan was inversely proportional to temperature. This was expected because it is well known that developmental rate of poikilothermic organisms is strongly affected by temperature, as has been observed many times and analyzed by Collinet et al. (2015). In contrast, it is noteworthy that relative humidity had an unimportant effect on the survival curves of R. indica, even though it ranged from 30 to 90 %. Moutia (1958), Hoy et al. (2006), Taylor et al. (2012), Prabheena and Ramani (2014), among others, have noted that R. indica populations naturally diminish during the rainy season, when relative humidity is obviously higher. This leads us to postulate that not relative humidity, but rather the direct physical action of rainfall kills many mites. Another important mortality factor could be epidemics of pathogenic fungi infecting R. indica, which are prevalent during the rainy season as has been observed by Carrillo et al. (2012b) and Colmenarez et al. (2014). All the above data show that relative humidity, within the experimental range, had no significant impact on R. indica development and reproduction.
Several studies present values on the duration of R. indica developmental stages, as well as on egg-to-adult duration. Such data are not totally comparable with those of our work because temperature, humidity or host plants were different, or simply because the environmental conditions were not specified in those studies. However, they provide useful information. Examples are Moutia (1958), Hoy et al. (2006) and Vásquez et al. (2015). In all these studies, the time elapsed from egg to adult varied between 20 and 38 days, coinciding with our research (Tables 2 and 3). Using the above studies as a reference, it can be determined that R. indica has a developmental time typical for Tenuipalpidae, considerably longer than that of the closely related family Tetranychidae, where the lapse from egg to adult ranges from six to ten days (Crooker 1985). The family Tenuipalpidae has traditionally been considered less important than the family Tetranychidae because tenuipalpids rarely attain high infestations, partially attributed to their slow development (Jeppson et al. 1975).
Because survival curves, duration of developmental stages and oviposition in R. indica were affected by temperature, the parameters of population increase were consequently related to temperature and to a lesser extent to relative humidity. The highest values of fecundity indices (Ro and λ) were observed when mites were reared at 27.5 °C, whereas the lowest values corresponded to 22.5 and 30 °C, in all cases 40-50 % RH. Relative humidity above and below 40-50 % resulted in lower fecundity indices. The intrinsic rate of population increase (rm), whose value is affected by all the above indices, was equally the highest at 27.5 °C, 40-50 % relative humidity.
These values highlight R. indica´s affinity to warm climates and its tolerance to wide variations in relative humidity. Field observations of this mite have repeatedly shown that its infestation increases during the warmest part of the year and its world distribution is pantropical even after settling in America (Peña et al. 2010; CABI 2016).
Vasquez et al. (2015) estimated an rm of 0.166 for R. indica feeding on coconut leaves. This value is notably higher than that estimated in our work (0.0856, Table 4). Because the authors of the cited study do not give details on how they calculated the parameters of population increase, it is difficult to interpret the difference. A possible explanation could be that in our study the parameters of population increase were estimated assuming that 80 % of all the specimens were females.
The rm estimated for R. indica is low compared with those from the tetranychid species; for example, for Tetranychus cinnabarinus (Boisduval, 1867) it was 0.20 (Peralta and Tello 2011). It is also lower than values estimated for other tenuipalpids; Kennedy et al. (1996) estimated rm = 0.127 for B. phoenicis, while Feres et al. (2010) found rm = 0.06 for T. heveae reared on a susceptible clone of Hevea brasiliensis (Willd ex. A. Juss) (Malpighiales: Euphorbiaceae).
All the above data show that R. indica has a developmental rate and fecundity similar to those of other tenuipalpids, resulting in similar values of Ro, λ and rm. It is then surprising that this mite reaches very high field infestations on coconut palm (Kane et al. 2012), its main host and where it was reared during the current study, contrasting with other tenuipalpids which are commonly present in low populations on their respective hosts (Jeppson et al. 1975). A possible explanation for such high R. indica infestations could be that in its new distribution areas in America effective natural enemies have not had enough time to adapt to their new prey. In addition, R. indica seems to have a defense mechanism against predators; it secretes a liquid that accumulates as droplets on the tips of its dorsal setae; Carrillo et al. (2012b) suggest that it is a repellent.
Finally, the proportion of R. indica males to females may also impact its capacity of population increase. In other species of Tenuipalpidae, such as Brevipalpus spp., practically all specimens are potentially fertile females (Helle et al. 1980; Kennedy et al. 1996) that contribute to population increase. The proportion of R. indica males is higher (Moutia 1958; this study). Given that each male can mate with three to six females, a proportion of 20 to 30 % males is sufficient to fecundate all the females present.