Introduction
In vitro embryo production (IVEP) has been prominent over the years, especially for domestic animals, such as cattle (Boruszewska et al., 2016). Nevertheless, IVEP its efficiency is limited; it can generate about 30-50% of viable blastocysts (Gonçalves et al., 2007; Pereira et al., 2010). Thus, improvements are required in the culture conditions of various stages of IVEP, such as oocyte recovery, in vitro maturation (IVM) of selected oocytes, and fertilization (IVF) and development (IVD) of presumed zygotes to blastocyst stage to be transferred or cryopreserved (Varago et al., 2008).
These initial stages of oocyte recovery and maturation are of great importance for better results, as the quality of cumulus-oocyte complexes (COCs) obtained determines the later stages of the technique (Santos et al., 2016; Santos et al., 2017). Therefore, to ensure nutrition and energy support during in vitro oocyte development, the media requires supplementation with external protein sources. Commonly used protein sources are fetal bovine serum (FBS) and bovine serum albumin (BSA) (Ambrogi et al., 2017).
FBS is an undefined source of protein, consisting of a combination of proteins, growth factors, hormones, vitamins, and other nutrients present in the blood plasma (Van Der Valk et al., 2010; Leivas et al., 2011). In contrast, BSA is a purified protein present at high concentrations in blood plasma, with high capacity and affinity for binding with other biomolecules (Muñoz and Mischler, 2015). Despite extensive use, the concentration of FBS and BSA in the recovery and maturation media varies among protocols, whether used alone or in combination (Sugimura et al., 2018). Therefore, establishing the ideal concentration according to the influence on oocyte quality and maturation rate can contribute to increasing the success of IVEP.
Thus, the present study aimed to evaluate the quality of immature bovine oocytes recovered and selected in medium containing different concentrations of FBS (10 vs. 20%) and BSA (5 vs. 10%), as well as to analyze the effect of these protein sources on the IVM of bovine oocytes (FBS: 5 vs. 10%; BSA: 0.4 vs. 0.8%).
Materials and Methods
Ethical considerations
The study was conducted according to the Institutional Committee on Animal Use at Universidade Federal Rural do Semi-Árido, Mossoró, Rio Grande do Norte, Brasil. (CEUA/ UFERSA, no. 23091.001069/2015-79). All reagents used were from Sigma-Aldrich® (Sigma, St Louis, MO, USA), except where indicated.
Study design
The study was divided into two stages. In Stage 1, protein sources were added in the recovery medium and in Stage 2 in the IVM medium. In the first stage, oocytes were obtained in recovery medium containing phosphate buffered saline (PBS) solution, and three experiments were performed according to the following supplements: (R1) 10 vs. 20% FBS (Gibco, Carlsbad, Kentucky, EUA); (R2) 5 vs. 10% BSA; and (R3) was the best combination that resulted from experiments R1, R2, and the combination of FBS+BSA (5+5%). Thus, the best results were defined according to the highest recovery rate, proportion of viable oocytes based on morphology, and proportion of viable oocytes based on brilliant cresyl blue (BCB) in each experiment. Additionally, a high percentage of viable oocytes were considered more significant during the evaluation of the results. After recovery, oocytes were evaluated for morphological appearance and BCB assay. In the second stage, we aimed to evaluate protein sources in the IVM medium; three experiments were conducted to compare the following: (M1) 5 vs. 10% FBS; (M2) 0.4 vs. 0.8% BSA; and (M3) best results from experiments M1, M2 and the combination of FBS+BSA (5+0.8%). Thus, the best results were defined according to better rates of expansion and viability of cumulus cells (CCs), presence of the first polar body (1PB) and metaphase plate (MII) for each experiment. For this purpose, oocytes were recovered in TCM199 supplemented with 10% FBS, 40 μg/mL gentamicin sulfate, and 0.2 mM sodium pyruvate. They were subjected to IVM and evaluated for the expansion and viability of CCs, the presence of the 1PB, and MII.
Ovarian collection and oocyte recovery
Bovine ovaries (n = 735) were collected at a local slaughterhouse (Mossoró, Rio Grande do Norte, Brazil). Immediately after slaughter, the ovaries were transported in saline solution (NaCl, 0.9%) at 35 °C for up to 30 min. Once in the laboratory, follicular aspiration was performed with the aid of 21G needles coupled to 5 mL syringes for 10-20 min. Thus, only visible follicles on the ovarian surface, with a diameter of 2-8 mm, were counted and aspirated. The follicular fluid obtained was kept at rest for 15 min for sedimentation of the oocytes. The time between ovarian collection and oocyte recovery was approximately 60 min.
Morphological evaluation and bright cresyl blue assay
The recovered COCs were morphologically classified as either viable (one or more layers of CCs and homogeneous cytoplasm) or non-viable (less than one layer of CCs and heterogeneous or degenerated cytoplasm), according to Santos et al. (2016).
For the BCB assay, oocytes selected by morphological criteria were washed in PBS and incubated in drops of BCB diluted in PBS (26 μM) for 60 min at 38.5 °C. Then, under stereomicroscope (Bel Photonics, Monza, BM, Italy), oocytes with blue staining in their cytoplasm were considered BCB+ and more competent. Those with unchanged cytoplasm were considered BCB- and less competent. This test is based on the ability of the enzyme glucose-6-phosphate dehydrogenase (G6PDH) to reduce BCB from blue to colorless in growing oocytes, whereas in oocytes grown at low activity the enzyme allows them to remain blue (Maia et al., 2017).
In vitro maturation (IVM) and evaluation of mature oocytes
In Stage 2, after oocyte retrieval, 20-30 morphologically classified as viable oocytes were transferred to droplets (covered with mineral oil) of the IVM medium consisting of TCM199 supplemented with 100 μM cysteamine, 0.2 mM sodiumm pyruvate, 40 μg/mL gentamicin sulfate, 10 μg/mL FSH/LH (Pluset®, Callier, Buenos Aires, ARG), and a protein source. The IVM was performed in petri dishes (Corning, Corning, NY, USA) at 38.5 °C in a humid atmosphere with 5% CO2 for 24 h.
After IVM, oocytes with expanded CCs, identified under stereomicroscope, were considered mature and those that did not show expansion were considered immature. To evaluate viability of CCs, the oocytes were denuded by repeated pipetting and the resulting cell suspension was stained with trypan blue (0.2%). The cells were counted in the four quadrants at the edges of a Neubauer chamber, where blue and non-stained cells were considered dead and live, respectively.
After CC removal, the oocytes were morphologically evaluated for the presence of 1PB under stereomicroscope. Structures showing the 1PB were considered mature. Furthermore, the nuclear stage of the oocytes was evaluated by fluorescence microscopy. For this, oocytes were fixed in paraformaldehyde (4%) and stained with Hoechst 33342 (10 μg/ mL) for 15 min. Only oocytes with nuclear stage in metaphase II were classified as mature.
Statistical analysis
Recovery rates (oocytes recovered of the total of aspirated follicles), oocyte quality (viable oocytes of the total oocytes recovered), and maturation rates (oocytes showing expanded CCs, presence of 1PB, and MII of total oocytes evaluated) were compared among the groups and the results were analyzed by the Fisher`s exact test (GraphPad Software INSTAT 3.06). The viability rate of CCs (live cells/total cells counted × 100) was analyzed by the Chi-square test. Data were expressed as mean ± standard error and considered different when p<0.05. In each experiment, five replicates were used.
Results
Stage 1 (Table 1) consisted of the evaluation of protein supplementation in immature bovine oocyte collection media. A total of 266 ovaries lead to 1.978 oocytes, with a mean of 7.4 oocytes retrieved per ovary. Thus, in experiment R1, 10% FBS was superior (p<0.05) to the 20% FBS group for morphological evaluation, and lower in the BCB test. Nevertheless, despite the superior result in the BCB test for 20% FBS, this occurred due to a higher amount of morphologically non-viable oocytes. In experiment R2, 10% BSA presented better morphological results compared to 5% BSA (p<0.05). Nevertheless, the two BSA concentrations showed statistically similar results in the BCB assay. Thus, 10% FBS and 10% BSA were used in R3.
In the latter experiment, 10% FBS and 10% BSA were compared to 5+5% FBS+BSA combination. The 10% BSA group had higher recovery rate than the other groups. However, the combination of protein sources obtained higher number of morphologically viable oocytes. In contrast, 10% FBS medium showed higher percentage of viable oocytes using the BCB assay. Therefore, supplements that achieved positive results for oocyte quality, that is, FBS+BSA (5+5%) combination and 10% FBS, were considered better.
Table 1 Evaluation of several concentrations of protein supplements in bovine immature oocyte recovery medium (R): Recovery 1 (10 vs. 20% FBS), R2 (5 vs. 10% BSA), and R3 (combination of FBS+BSA (5+5%) vs. 10% FBS vs. 10% BSA).
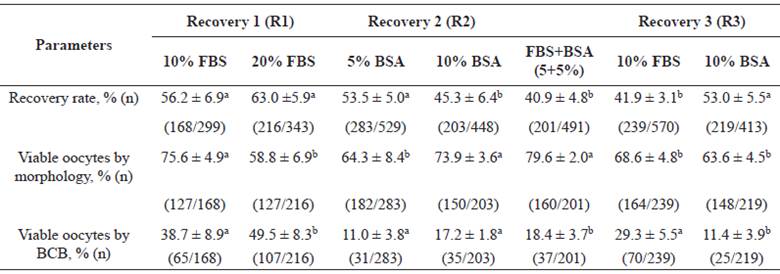
Values are means (%) ± Standard Error (n). Different superscript letters (a, b) in each experiment (R1, R2 and R3) within the same row indicate significant difference (p<0.05).
Stage 2 (Table 2) aimed to evaluate these supplements in the IVM media. A total of 469 ovaries resulted in 1.040 morphologically viable oocytes (2.2 viable oocytes per ovary). As for experiment M1, there was no difference (p>0.05) between 5 and 10% FBS groups regarding expansion and viability of CCs, presence of 1PB and MII.
Therefore, 5 and 10% FBS were evaluated in the M3 experiment.
In experiment M2, 0.8% BSA was superior (p<0.05) only for viability of CCs compared to 0.4% BSA. 0.8% BSA was used in the subsequent experiments. In experiment M3, 5, 10% FBS, 0.8% BSA and the FBS+BSA (5+0.8%) combination were similar (p>0.05) for all parameters.
Table 2 Evaluation of several concentrations of protein supplements for in vitro maturation medium (IVM) of bovine oocytes: Maturation 1 (5 vs. 10% FBS), M2 (0.4 vs. 0.8% BSA), and M3 (combination of FBS+BSA (5+0.8%) vs. 5% FBS vs. 10% FBS vs. 0.8% BSA).

Values are means (%) ± Standard Error (n). Different superscript letters (a, b) in each experiment (M1, M2 and M3) within the same row indicate significant difference (p<0.05). 1PB: first polar body. MII: metaphase II.
Discussion
Several concentrations of two protein sources (FBS and BSA) were evaluated for bovine oocyte recovery and IVM media. In the recovery medium (R1), 10% FBS presented the best result for morphological quality in comparison with 20% FBS. This demonstrates that even at low concentration FBS is able to provide good nutrition to the oocytes during the recovery while maintaining high quality for IVM. In contrast, a better effect of BSA (R2) was observed at the highest concentration tested (10%), probably due to the simpler composition of this supplement. Nevertheless, in the third experiment (R3), when comparing 10% FBS, 10% BSA, and FBS+BSA (5+5%) combination, the most favorable supplement mix for oocyte quality were 10% FBS and FBS+BSA (5+5%) combination.
In general, FBS concentration in the oocyte recovery media is variable. Chasombat et al. (2015) used 10% FBS in bovine oocyte retrieval medium obtaining good blastocyst rate (30.9%) at the end of IVF. On the other hand, Chen et al. (2016) used a 2% FBS during the recovery and reached 23.0% blastocysts after 8 days of culture of the embryos generated. Furthermore, with respect to BSA, previous studies show variations in the quantities used. As an example, Ulloa et al. (2014) compared bovine oocyte harvesting methods using 0.1% BSA and obtained 79.8% maturation after follicular aspiration. In the study by Carrocera et al. (2016), bovine oocyte recovery medium was supplemented with 0.4% BSA and, after IVF, up to 37.5% blastocyst was obtained. In other species, such as pigs and mice, the use of 0.4% (Galeati et al., 2010) and 10% (Dinopoulou et al., 2016) BSA has also been reported. The conjugation of FBS+BSA (5+5%) in the recovery medium may be an interesting supplement since it allowed a great nutritional contribution to the oocytes and showed a better rate of BCB+ oocytes.
The use of the BCB assay for the selection of viable bovine oocytes proved to be an efficient method by allowing the semi-quantification of G6PDH enzyme, indicative of the stage of oocyte development. According to Ashry et al. (2015), oocytes classified as BCB+ in the recovery stage are more competent for embryonic development. From this, it is suggested to perform this in conjunction with morphological evaluation since morphologically non-viable oocytes for IVEP can also be stained with the dye.
In the IVM stage, different concentrations of FBS and BSA were evaluated separately and associated; then, the best results were compared with a group containing both supplements. Regarding FBS, both tested concentrations (5 and 10%) showed no difference. In the experiment with BSA (M2), 0.8% was superior compared to 0.4% regarding CCs viability. Previously, Ali and Sirard (2002) compared different protein sources during maturation. They verified the effect of free fatty acid BSA (0.8%), isolated BSA (0.8%), chicken egg albumin, polyvinylpyrrolidone (PVP) (0.8%), and 10% FBS in the media, and obtained low maturation rate (44.0%) when using 0.8% BSA, but achieved the best results using PVP supplementation, which is different from the results of the present study.
The cells surrounding the oocyte are of great importance for oocyte maturation, as they have gonadotrophin receptors (FSH and LH), communicate, and exchange nutrients and molecules important for oocyte development (Crocomo et al., 2011). Thus, the evaluation of CCs is important for efficient IVM, because the viability parameter reveals the normal functioning of oocyte communication and media efficiency, and the expansion is related to cytoplasmic maturation (Araújo et al., 2014).
In the M3 experiment, concentrations of 5 and 10% FBS, 0.8% BSA, and the FBS+BSA (5+0.8%) combination were compared. According to Del Collado et al. (2014), low FBS concentrations in IVM can generate results similar to those obtained under high FBS concentrations. Thus, the lowest FBS concentration (5%) was used in the medium containing the conjugation of the protein sources. After evaluating CCs and nuclear stage, all groups presented similar results. This could be because the FBS and BSA concentrations have enough nutrients to ensure oocyte development. Furthermore, Ambrogi et al. (2017) compared the transport of bovine oocytes in media supplemented with 10% FBS or 0.6% BSA followed by IVM with 10% FBS and found no differences in MII rates, blastocysts, cryotolerance, levels of reactive oxygen species, and mitochondrial distribution (cytoplasmic maturation). This shows that the two supplements have similar efficiency.
The use of more than one protein source during the same stage of IVEP has been reported by other researchers. Leivas et al. (2011) used a medium supplemented with BSA and FBS to culture bovine embryos and obtained up to 43% blastocyst rates. Del Collado et al. (2015) compared FBS+BSA (5+0.6%) conjugation during IVM where 89.6% maturation was reached in the presence of 1PB, a result very similar to that obtained in the present study (89.7%). These authors suggest that the presence of only BSA in the IVM medium is less effective compared to a medium containing both protein sources. Therefore, it is believed that supplementation of FBS together with BSA presents greater protein supply for COCs development, by combining positive characteristics of both supplements. However, when used alone at suitable concentrations, FBS and BSA show positive and similar results and can be used for bovine oocyte IVM.
In conclusion, supplementation with FBS+BSA (5+5%) in combination and 10% FBS is beneficial for the recovery medium, allowing a greater number of morphologically viable oocytes and BCB assay. At the IVM stage, only the addition of 0.4% BSA would not be of interest as a supplement in IVM medium for bovine oocytes. Thus, FBS (both 5 and 10% levels) and BSA (0.8%) can be used alone or in combination with a similar positive effect on oocyte maturation.














