Introduction
Natural and synthetic growth promoters are used in poultry diets to improve growth efficiency. Growth promoting antibiotics have been used to increase growth efficiency and minimize mortality by altering the microbiota in the intestinal tract of healthy chickens, resulting in improved growth efficiency (Sabah-Abdulameer et al., 2022). Nevertheless, bacterial resistance to antibiotics in humans is a problem that demands good planning and comprehensive research (Chattopadhyay, 2014). Many countries have banned the use of antibiotic growth promoters (AGP) in animal feeds, which highlights the complexity of the issue (Cuong et al., 2021). Numerous feed additives, such as herbal plants, beneficial bacteria, oligosaccharides, essential oils (Al-Sultan et al., 2016; Sabah-Abdulameer et al., 2022), and nanoparticles (Saad Ibrahim et al., 2022) are used aiming to replace AGP. Nanoparticles are used as feed additives for farm animals, affecting the physiological status of some organs (Saad Ibrahim et al., 2022). Furthermore, nanovitamins and minerals are used in both animal nutrition and the medical field (Swain et al., 2000). The use of antimicrobial, antifungal, and immune induction methods can increase growth efficiency in broiler chickens (Hassan-Pour et al., 2015; Patra and Lalhriatpuii, 2020).
It is also known that selenium (SE) can improve the immune status of avian and animal species (Ebeid et al., 2013; Rayman, 2004). Pečjak et al. (2022) reported that SE enhances the growth factor and meat characteristics, while reducing stress and mortality in poultry. However, the use of SE in poultry depends on its chemical form and combination with other compounds (Surai et al., 2018). The mixture of SE and tocopherol (vitamin E) is commonly used in poultry feed (Surai, 2002a). Recently, the interest in nano-SE has increased because of its catabolic efficiency, high metabolic ability, and low toxic effects compared with selenite in poultry (Wang et al., 2009), mice, rabbit (Wang et al., 2007), rat (Jia et al., 2005), and ovine (Shi et al., 2011a; Shi et al., 2011b). While there is enough evidence on the role of SE and tocopherol on growth performance, less attention has been paid to immune induction in broilers fed nano selenium.
Tocopherol protects biological membranes and heals injured cells (Sharifi-Rad et al., 2020). The mixture of tocopherol and SE can stimulate growth production and immunity (Singh et al., 2006). Moreover, the mixture of tocopherol and SE can increase blood IgG and IgM levels (Et-Shenawy et al., 2015). Tocopherol (150 IU/kg) and SE (0.06 ppm) seem to increase antibody response in broiler chickens, with cell-mediated immunity response when fed 300 IU/kg tocopherol and 1 mg/kg SE (Lara et al., 2013). Although it is well-known that the interaction between SE and tocopherol can enhance growth and the antioxidant system, few studies have evaluated the effect of the interaction between nano tocopherol and SE in broilers. Therefore, the present study aimed to evaluate the effects of incremental levels of a mixture of nano α-tocopherol acetate and SE on growth performance, immune response, carcass traits, and intestinal histomorphometry of broiler chickens.
Materials and methods
Ethical considerations
The Ethics Committee for Poultry Research at Al-Qasim Green University in Iraq approved the study protocol (act: Alqas-rec.2021-Jun-EA98737).
Nanoparticles
A powder mixture of nano α-tocopherol acetate (vitamin E) 10% and sodium selenite (0.1% SE) was used (Rheinvet Animal Health GmbH®, Neuwied, Germany).
Animal and dietary management
A total of 240 (50% males, 50% females) one-day-old Ross 308 broiler chickens were studied during a 42-days trial. The chickens (45 g±5 average weight) were randomly distributed into four treatment groups, each containing three pens (n=20 per pen). Four doses of nano α-tocopherol acetate and SE (0, 5, 7, and 10 mg/kg) were included in the basal diet for 42 days. The feeding program consisted of starter feed from 1 to 21 d, and grower feed from 22 to 42 d. The experimental diet formulations were formulated according to the nutritional requirements of broiler chickens based on the 1994 Nutrient Requirements of Poultry (NRC; Table 1).
The chickens were randomly divided into four groups, as follows: Treatment 1 (control group): basal diet (BD) without any feed additive; Treatment 2: BD plus 5 mg/kg of nano α-tocopherol acetate and selenium (NTS); Treatment 3: BD plus 7 mg/kg NTS; and Treatment 4: BD plus 10 mg/kg NTS. Litter was used for keeping the birds under standard conditions according to the Ross broiler guidelines. Water and feed were provided ad libitum. In the first week, the temperature was 33±1 °C, and was reduced to 27 and 24 °C in the second and third weeks, respectively. The temperature was kept at 23±1 °C from the beginning of the fourth week until the final day of the experiment.
Table 1 Feed ingredients and chemical analysis (g/kg) of basal feed in broiler chickens supplemented with NTS for 42 days.

*Premix per kg of diet: Vit D3 3,500 IU; Vit B6 (riboflavin) 3.44 mg; Menadione 2.29 mg; Niacin 40.17 mg ; Vit E (α-tocopherol) 44.7 IU; Iron 120 mg ; Zinc 120 mg; Pantothenic acid 6.46 mg ; Pyridoxine 2.29 mg; Biotin 0.05 mg; Folic acid 0.56 mg; Cyanocobalamin 0.05 mg; Vit A 12000 IU; Thiamine 1.43 mg; Copper 15 mg; Manganese 150 mg; Cobalt 0.4 mg; Selenium 0.3 mg; Iodine 1.5 mg.
Growth performance
Feed intake (FI), average body weight (ABW), and body weight gain (BWG), in each pen were measured weekly. Feed conversion ratio (FCR) was measured for all the experimental period by dividing FI by BWG (Mohseni et al., 2021).
Vaccination program
Each group received ocular vaccination against Newcastle disease (ND) (Nobilis® ND LaSota, Intervet Co, Millsboro, USA) and IBV (Hitchner IB) at the first and tenth days of age. Infectious bursal disease (IBD) vaccine was given by eye-drop on day 14. Avian influenza (AI) and ND vaccines were intramuscularly administered on the first day of life (Jordan Bio Industries Co, Amman, Jordan) according to the Ross broiler recommendation.
Immune response
On day 34, blood was drawn from the jugular vein of two chickens per replicate (one male and one female) and centrifuged at 4500 rpm for 5 min. The sera were isolated and stored at -20°C until immunological analysis. The titers were determined using a commercial enzyme-linked immunosorbent assay (ELISA) kit "(ID.VET.BASELINES-MENA)" according to the manufacturer’s protocol. Mean antibody titers were read according to Fanar et al. (2020).
Carcass traits
Internal organs and dressing percentage. On day 42, two chickens (one female and one male) were randomly selected from each replicate and prevented from feeding for 6 hours. The chickens were weighed, slaughtered, scalded, plucked, eviscerated, and washed. The weight of the birds was recorded before slaughtering and again after evisceration. Carcass parts (breast and thigh of warm carcass), immune organs (bursa of Fabricius and spleen), and other organs (heart, liver, and gizzard) were weighted. Organ weight was calculated as percentage of carcass weight (Attia et al., 2020).
Intestinal histomorphometry
To assess intestinal histomorphometry, about 2.5 cm of the middle part of the jejunum (one bird/pen) was excised and washed with normal saline. The samples were fixed in a 10% solution of neutral buffered formalin (NBF). Increased concentrations of ethyl alcohol were used to dry the samples. Next, jejunum samples were dehydrated using a tissue processing machine (Leica, ASP300, Tokyo, Japan). Intestinal samples were cleared with xylene and embedded in a Leica EG 1160 paraffin embedding station (Leica EG, 1160, Bensheim, Germany). The hematoxylin (Gurrs, London, UK) and eosin (Chroma Gesellschaft, Münster, Germany) was used to stain the samples (4-5 mm thick) and then examined under light microscope (100X). Depth of invagination between adjacent villi (crypt depth) and height from the tip of the villi to the villi crypt junction (villi height) was measured with an image analyzer (Diagnostic Instruments Inc, Sterling Heights, Michigan USA) according to protocol reported in our previous study (Abdulameer et al., 2021).
Results
Growth performance
Table 2 shows growth performance results. Body weight (BW) and cumulative body weight gain (BWG) significantly increased with increasing NTS levels from 5 to 10 mg/kg (P<0.05). The highest BW (2,725 g) and BWG (2,666 g) were recorded on day 42 in the group receiving 7 and 10 mg/kg NTS. Final feed intake (g/bird) did not change (p>0.05) with 5 and 7 mg/kg NTS during the different phases of the experiment, but it significantly increased with 10 mg/kg NTS compared with the control group (p<0.05). Cumulative FCR was improved by 5 and 7 mg/kg NTS compared to the control group. Moreover, chickens receiving 10 mg/kg NTS showed lower FCR value (1.61) compared to the control (1.81) during the study (p<0.05) (Table 2).
Table 2 Growth performance of broilers fed α-tocopherol acetate and selenium.
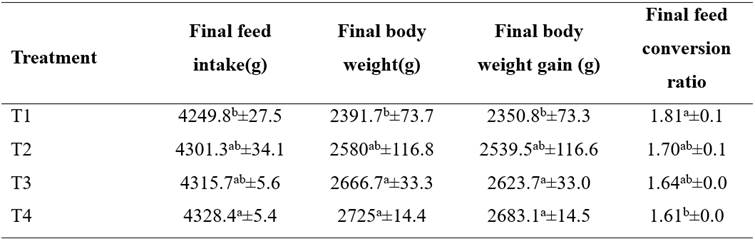
* T1: Basal diet (bd) without additives; T2: bd + 5mg/kg NTS; T3: bd + 7mg /kg NTS; and T4: bd + 10 mg/kg NTS. Statistical differences (p<0.05) between experimental groups within columns are indicated by different superscript letters (a, b). All values are presented as mean values ± SEM (n=4).
Table 3 Effect of NTS on carcass traits and internal organs of broilers.

*T1: Basal diet (bd) without additives; T2: bd + 5 mg/kg NTS; T3: bd + 7 mg/kg NTS; and T4: bd + 10 mg/kg NTS. Statistical differences (p<0.05) between experimental groups within columns are indicated by different superscript letters (a, b). All values are presented as mean values ± SEM (n=4).
Effect of NTS on carcass traits and internal organs
The NTS diets (7 and 10 mg/kg) significantly increased warm carcass and organ weight compared to the control (p<0.05) (Table 3). Liver, gizzard, and heart weight increased by using NTS at 10 mg/kg (p<0.05). Additionally, the weight of thigh and pectoral muscle increased with increasing NTS doses compared to the control (p<0.05). However, the weight of bursa of Fabricius and spleen did not change (p>0.05; Table 4).
Immune response
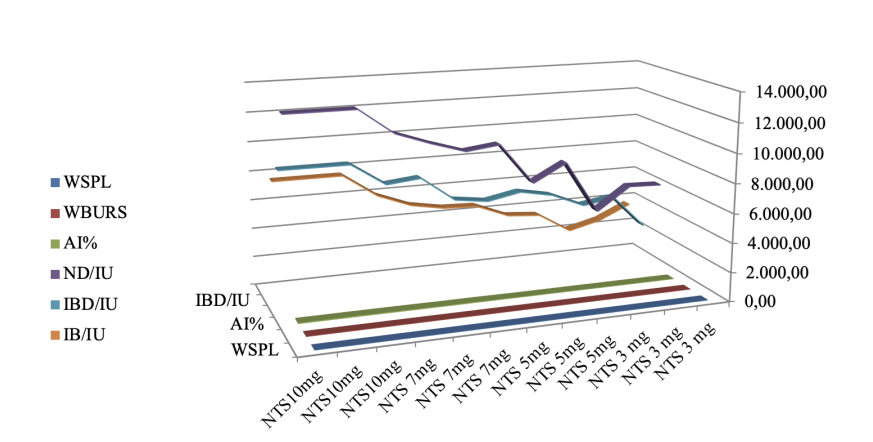
At day 34, a significant increase (p<0.05) was observed in antibody titers against IBV, IBD, ND, and AI in the group receiving NTS diet (5, 7, and 10 mg/kg; Table 5 and Figure 1).
Table 5 Effect of NTS diet on immune titers of broiler chickens after 34 days.
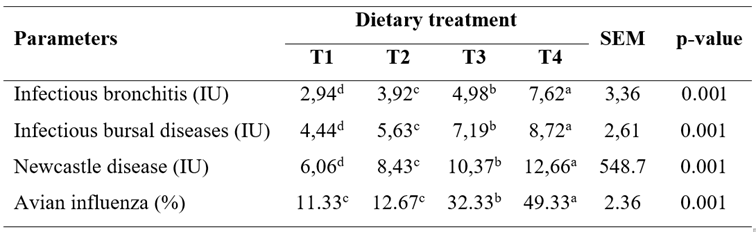
T1: Basal diet (bd) without additives; T2: bd + 5 mg/kg NTS; T3: bd + 7mg/kg NTS; and T4: bd + 10 mg/kg NTS. Statistical differences (p<0.05) between experimental groups within rows are indicated by different superscript letters (a, b, c, d). All values are represented as mean and standard error of the mean (SEM; n=4).
Intestinal histomorphometry
No changes were observed in villi height and width in the jejunum of chickens receiving NTS diet compared to the control. The most significant histomorphometry changes were related to villi height (VH): crypt depth (CD) ratio (p≤0.05; Table 6 and Figure 2).
Table 6 Effect of NTS diet on jejunum histomorphometry of broilers after 42 days.
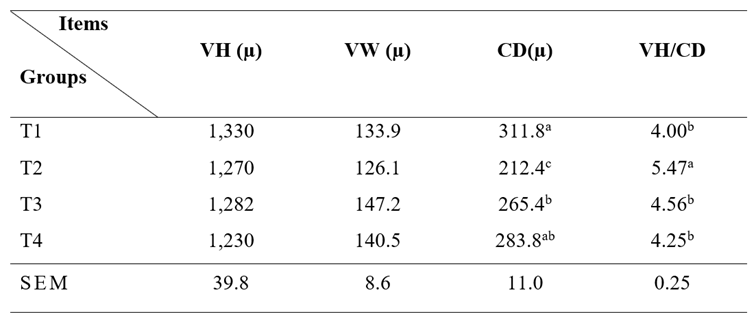
T1: Basal diet (bd) without additives; T2: bd + 5 mg/kg NTS; T3: bd + 7 mg/kg NTS; and T4: bd + 10 mg/kg NTS. VH: Villi height, VW: Villi width, CD: Crypt depth. Statistical differences (p<0.05) between experimental groups within columns are indicated by different superscript letters (a, b, c). All values are presented as mean and standard error of the mean (SEM; n=4).
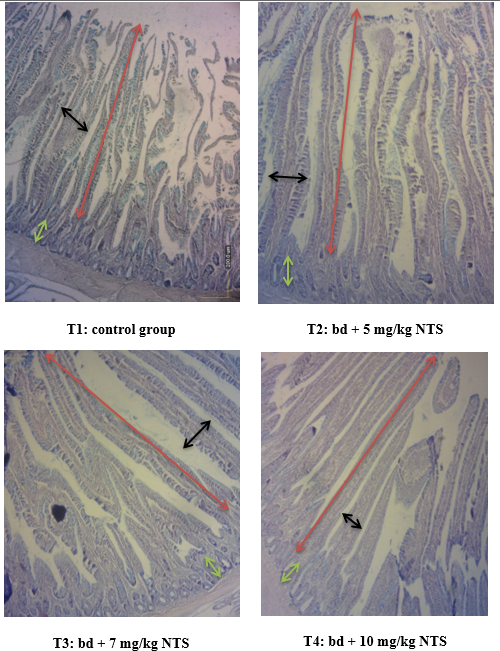
Figure 2 Histological features in jejunum of broiler chickens stained with haematoxylin and eosin (H&E) 100X. Control, birds fed basal diet (T1), basal diet containing 5 mg/kg of NTS (T2, basal diet containing 7 mg/kg NTS feed (T3); basal diet containing 10mg/kg of NTS (T4). Red line = villus height, black line = villus width, green line = crypt depth.
Discussion
This study showed that NTS had a positive synergistic effect on growth (BW, BWG, FI, and carcass traits) and health (intestinal histomorphometry, immune response, and blood profile) of broilers. The NTS feed additives have antioxidant compounds that protect chickens from oxidative deterioration (Cui Zhu et al., 2022). Several studies have suggested that NTS can inhibit (Ahmadi and Kurdistani 2010; Mancapecjak et al., 2022) or stimulate growth (Harsij et al., 2020; Olla et al., 2021). In the present study, growth factors improved with 7 and 10 mg/kg NTS. These findings are consistent with the results of Salahuddin et al. (2017), reporting that vitamin E (100 mg) and SE powder (0.22 mg/kg) improved live BW and BWG. The results of Dosoky et al. (2021) were also in line with the previous studies (2, 4, and 8 ppm of SE). This improvement may be due to the antioxidant effect of NTS on intestinal status, improved gut health, and absorption, because a healthy gut increases nutrient absorption, weight gain, and feed consumption in NTS-fed broilers (Ghazi et al., 2016). Also, NTS has anti-inflammatory effects because it acts as a free radical scavenger affecting the mechanism of inflammation and healing (Alicalik et al., 2022). Furthermore, vitamin E and SE stimulate gut enzymes, which is another possible reason for the growth stimulating effect of NTS. The improvement in health status and immune responses of chickens fed NTS might be attributed to improved cellular functions and productivity with little use of nutrients (Fondevila, 2010). The best growth observed can be attributed to lower energy expenditure on feather growth (Edens et al., 2001; Roozbeh-Shabani et al., 2019) since growth of feathers is highly demanding in energy (Yoon et al., 2007).
In contrast, some researchers reported that NTS supplementation did not affect broiler production (Swain et al., 2000; Habibian et al., 2014; Pompeu et al., 2018). These inconsistent results are possibly attributable to the structure of SE used in the study, where SE activity depends on its biochemical form. Surai et al. (2002b) showed that SE nanoparticles is better than organic SE; the effect of SE depends on its synthesis, doses, and the route of administration. The increase in feed consumption of broilers fed high NTS doses (7 and 10 mg/kg) may be attributed to the health status of chicken’s gut because of the antioxidant properties of vitamin E and/or SE.
In contrast, Salahuddin et al. (2017) revealed that broiler feed intake did not change by feed additive of vitamin E and SE. Also, Yuming et al. (2000) stated that feed consumption was not affected by vitamin E and SE supplements. Likewise, Arrieta et al. (2002) reported that vitamin E and/or SE did not affect feed consumption.
The highest FCR was observed in birds that received NTS, which can be attributed to the significant increase in feed intake and weight gain. Our results are also in line with the study by Choct et al. (2004), who increased SE doses improving the FCR response of chickens. Ziaei et al. (2013) reported that FCR of broilers improved with the addition of vitamin E and SE. These findings differ from those reported by Habibian et al. (2014) in which vitamin E and SE did not affect FCR during the whole experimental period. Ryu et al. (2005) and Edens (2000) discussed that vitamin E and SE are less palatable, which decreases feed consumption and FCR.
In our study, the enhancement in dressing percentages, carcass parts, and weight of the internal organs in chickens receiving several levels of NTS agreed with the study by Mahmoud H El-Deep et al. (2016). Furthermore, dietary addition of nano SE increased muscle and plasma vitamin E content under hot environmental conditions. This finding is congruent with the study by Ahmadi et al. (2018), in which NTS (4, 8, and 12 mg/kg) increased the relative weight of organs and carcass with no effect on immune organs. Similar to our results, Felehgari et al. (2013) reported that the weight of internal organs increased, but the weight of some organs did not change using several SE doses. The improvement observed in carcass parts (pectoral and thigh muscle) of chickens receiving nano SE agrees with previous findings (Konieczka et al., 2015; Ahmadi et al., 2018).
Crypt depth is a major indicator of health in chickens (Uni et al., 1995). The increased ratio of villi height: crypt depth (VH/CD) can enhance nutrient absorption and improve gastrointestinal tract secretion (Ege et al., 2019). Also, VH/CD ratio is positively related with enhanced turnover of epithelial cells. In our study, the mixture of SE and vitamin E did not affect villus height or width. Similarly, Aliyu Ibrahim Muhammad (2021) indicated that SE from yeast increased the VH/CD ratio in the small intestine (p<0.05), but it reduced crypt depth. Similarly, Ahmed et al. (2016) showed no effect on villi height in the ileum of chickens receiving dietary organic SE from yeast. In addition, VH/CD ratio enhanced by SE supplementation, and antioxidants and immune properties significantly improved (Tong et al., 2020).
We have seen how dietary NTS minimizes common diseases in Iraq. Typically, the addition of NTS (5, 7, and 10 mg/kg) stimulates the immune response. High doses of NTS were often more effective. In addition, NTS supplementation increased the immune response against AI, IB, IBD, and ND, but the immune organs (bursa of Fabricius and spleen) in the NTS group were not significantly affected. Several studies showed that nano SE affect antibody titers (Zhou and Wang, 2011; Liao et al., 2012). Since SE acts as an immunostimulant, SE deficiency leads to suppression of immune function due to reduced division of neutrophils and macrophages. This depression in cell division may be attributed to increased lipid peroxidation, which is followed by the accumulation of toxic compounds within the immune cells. The increase of toxic compounds in neutrophils leads to low immune function (Wen et al., 1998). Furthermore, a lack of SE interferes with the presence of nutrients in the bloodstream that are responsible for improving the immune system (Cao et al., 2002). Therefore, the combination of SE and vitamin E induces the immune response and cell proliferation. The oxidizing and peroxidative effects or the biological and toxic potential of SE depend on its chemical composition and structure. It has also been reported that SE nanoparticles are more efficient in terms of immune response, especially when combined with vitamin E (Zhang et al., 2008; Ahmadi et al., 2018).
Tocopherol and SE significantly increase antibodies titers after vaccination against ND, IB, IA, and IBD (Wang et al., 2007; Kumar et al., 2009). The ability of lymphocytes to divide is reduced due to the lack of SE in the feed. Therefore, lymphocyte production decreases, leading to decreased immune function. Peng et al. (2009) argued that bioactive SE is more effective in inducing lymphocyte cell division. Most researchers have argued that SE improves immune function via enhancing T helper cells and improving cytokine secretion (Burton et al., 1977).
The high immune titers after NTS treatment in the present study is also congruent with the studies by Payne and Southern (2005), and Funari Junior et al. (2012), who reported that nano SE in poultry feed enhance cytokine activity and immune response. Cytokines and immune complexes are increased with better nutrient and cell growth due to SE and vitamin E supplementation (Grivennikov et al., 2010). Vitamin E can increase antibody titers and cellular immune response in broilers; it can also be used as an immunostimulant in broiler chickens at a dose of 100 mg/kg (Babak Darabighane et al., 2017). In addition, vitamin E can improve the immune response against several viral diseases (Abdulwahid et al., 2016).
In conclusion, according to the results of this study, NTS (5, 7, 10 mg/kg) has a significant effect on growth, FCR value, carcass traits, and intestinal histomorphometry. Besides, NTS has a potential to improve the immune response of broiler chickens against common viral diseases. However, the existing data regarding NTS residues in meat are contradictory and need further research.