Introduction
There is a growing interest in the study of organometallic complexes derived from fused ring ligands such as pentalene, indacene and dicyclopenta dienyl naphthalene. These fused ring ligands are anti- aromatic in nature and belong to the 4npi electron system. Much of the interest is in the study of the electron delocalisation between the metal centres comprising of these ligands [1-7]. It was found that there is much greater electronic interaction in these systems due to the overlapping of the d-orbital of the metal and the p orbital of the ligand, evidenced by the spectroscopic and electrochemical characterisation [8]. Due to this interaction, a number of applications of these materials may be expected by appropriate design of the bridging groups for appropriate super exchange, combined with the use of two different metals with different spin and may lead to a ferrimagnet [9-10]. Several studies have centred on the use of bi nuclear metallocenes for third-order nonlinear optical properties. We can also imagine the second-order properties by using other species where the interaction between a metallocene donor and a metallocenium acceptor is important. Various research groups continued their effort in synthesising said organometallic compounds with novel electrical and magnetic properties [11-13]. For example, the Manriquez group have prepared the number of organometallic compounds with s-indacene as a spacer ligand and reported some interesting delocalisation properties [14-16]. Notwithstanding, to our knowledge there is no work reported on bi nuclear metal complexes with two s- indacene spacers.
A mono benzo analogue of pentalene has two isomers, s-indacene and as-indacene (Figure 1).It was found that the complexes derived from s-indacene exhibit a much higher degree of metal-metal interaction than the complexes derived from as- indacene. It was concluded that the geometry of the ligand plays an important role in the intermetallic interactions in these systems. Subsequently it was proved by virtue of Huckel's extended theoretical calculations [16].
In this paper we investigate the homo and hetero bimetallic complexes of substituted s-indacene in which two metal centres are sandwiched between the indacene spacer. We also report its electrochemical, Mossbauer and magnetic measurement results along with the DFT calculations.
Materials and methods
General
All reactions were carried out under inert nitrogen and in dry solvents. Iron(II) Chloride wasprepared in situ with standard procedure. The following materials were used as supplied commercially without further purification: anhydrous CoCl2, diethyl methyl malonate, KOH, HCl, n-butyl lithium (1.6 mol L-1), poly phosphoric acid and ethyl alcohol.
Instrumental
Bruker-400 (400 MHz and 100 MHz for proton and Carbon NMR respectively) was used to record the NMR spectra and chemical shift reported in ppm relative to TMS. Bruker Vector-22 FTIR spectrometer was used to record the FT-IR spectra using Nujal Mull sample between NaCl discs. Fisions instruments EA-1108 CHNS elemental analyser was used for the elemental analyses. Bio analytical Systems Voltammetric analyser (model CV-50w, version 2.3) was used for cyclic voltammetry measurements. Platinum or a glassy carbon disc were used as a working electrode and platinum wire as an auxiliary electrode. The auxiliary electrode was isolated from the bulk solution by a glass tube with a small-porosity glass frit at the end. Neutral aluminium oxide was placed on the frit and the tube was filled with a 0.1 mol L-1 solution of supporting electrolyte[N(Bu)4]BF4 (Bu=butyl); the reference electrode was an Ag/AgCl wire placed in a tube with a cracked glass bead at the end and containing aqueous tetra methyl ammonium chloride. The concentration of this solution was varied until the potential value was 0.0 Vvs the saturated calomel electrode (SCE). This electrode was located inside a Luggin capillary in the electrochemical cell. The solvent used was dichloromethane. All the measurements were carried out under nitrogen atmosphere at room temperature (25oC).
A constant acceleration Mossbauer spectrometer with 57Co/Rh source was used for the Mossbauer analyses of the samples. The c counts were collected in a 512 multi-channel analyser while the source was moving via triangular velocity waveform and, computer procedures were adopted for plotting and analysing the data. Velocity calibration was performed using 25 mm thick metallic Fe foil. The Mossbauer spectral parameters are given relative to this standard at room temperature. Mossbauer analyses were carried out at the UGC-DAE facilities at Indore, India.
Magnetic measurements were carried out with Quantum design (USA) Physical Property Measurement System (PPMS) with vibrating sample magnetometer (VSM) that has a temperature range from 1.9 to 400 K with a field range of 0 to 9 Tesla with sensitivity 1X10 -6 emu and oscillation frequency 40 Hz. All magnetic measurements were carried out at the UGC-DAE facilities at BARC, Mumbai, India.
The density functional calculations of the complex were performed using the Gaussian programme G09 and the Gauss view 6 with the 3-21G*(6d, 7f) basis sets. The frontier molecular orbital profile and the energy gap between the highest occupied molecular orbital (HOMO) and the lowest unoccupied molecular orbital (LUMO) were optimised. The natural bonding orbitals derived from the same fundamental sets.
Syntheses of ligands
The substituted s-indacene ligands, namely 2,4,6,8 teramethyl-1,5-dihydro-s-Indacene(L1) and 2,6 diethyl-4,8-dimethyl-1,5-dihydro-s-indacene (L2) were prepared in a good yield by reported methods [17].
Syntheses of Organometallic compounds
Synthesis of Bis (2,4,6,8 teramethyl-indacenyl) di Iron (1)
In a100 mL Schlenk flask, 0.5 g (0.0023 mol) of L1 was dissolved in tetra-hydrofuron (30 mL) and cooled to -78 oC. Under nitrogen atmosphere, n-butyl lithium in hexane (2 mol L-1, 2.37 mL) was added drop wise to L1, after complete addition it was allowed to warm to room temperature and allowed to stir for 1 h. Successively, anhydrous Iron(II) chloride (0.3 g, 0.0023 mol) dissolved in THF was charged (transferring via cannula) at -78 oC. After the complete transfer of FeCl2 the reaction mixture was allowed to warm to room temperature and stirred for 3 h. Solvent was evaporated under vacuum and the dark brown colour product was extracted with toluene until the extraction became colourless. The combined extracts were put together and evaporated to dryness. To this crude product, THF was added (20 mL) to dissolve and cooled to -78 oC. The precipitate was filtered and dried yielding 0.5 g. (67%) dark red microcrystalline product, sensitive to air.
1H NMR (C6D6, ppm) 1.36(s, 12H,-CH3) 2.44 (s, 12H, CH3, benzylic) 4.92 (s, 4H, CH) 5.22 (s, 4H, CH).
Elemental analysis, Calcd for C32H32Fe2: C 72.86, H 6.07; found: C 72.72, H 6.01.
Synthesis of Fe(2,4,6,8 teramethyl s-indacenyl) 2 Co (2)
In a 100 mL Schlenk flask, 0.5 g (0.0023 mol) of L1 was dissolved in tetrahydrofuron (30 mL) and cooled to -78 oC. Under nitrogen atmosphere n-butyl lithium in hexane (2 mol L-1, 1.19 mL) was added drop wise to L1; after complete addition it was allowed to warm to room temperature and stirred for 1 h. Successively, anhydrous Iron(II) chloride (0.15 g, 0.0012 mol) dissolved in THF was charged (transferring via cannula) at -18 oC. After the complete transfer of FeCl2 the reaction mixture was allowed to warm to room temperature and stirred for 2 h. This reaction mixture was again cooled to -78 oC and n-butyl lithium in hexane (2 mol L-1, 1.19 mL) was added drop wise. Then, we allowed to warm to room temperature and stirred for 1h. Anhydrous cobalt chloride (0.15 g, 0.0012 mol) in tetrahydrofuron (30 mL) was added and stirred for 4 h. Solvent was evaporated under vacuum and the dark brown colour product was extracted with toluene until the extraction became colourless. The combined extracts were put together and evaporated to dryness. To this crude product, THF was added (20 mL) to dissolve and cooled to -78 oC. The precipitate was filtered, dried yielding a dark brown product 0.4 g (61%).
1H NMfJRTCft, ppm) 0.29 (s, 6H, CH3) 1.31 (s, 6H,-CH3) 2.44 (s, 12H, CH3, benzylic) 4.92 (s, 4H, CH ) 5.25 (s, 4H, CH).
Elemental analysis, Calcd for C32H32FeCo: C 72.26, H 6.02; found: C 71.16, H 5.97.
Synthesis of Bis (2,6 diethyl-4,8-dimethyl-s-indacenyl) di cobalt (3)
In a100 mL Schlenk flask, 0.5 g (0.0029 mol) of L2 was dissolved in tetrahydrofuron (30 mL) andcooled to -78 oC. Under nitrogen atmosphere, n-butyl lithium in hexane (2 mol L-1, 2.37 mL) was added drop wise to L2. After complete addition it was allowed to warm to room temperature and allowed to stir for 2 h. Successively, anhydrous cobalt(II) chloride (0.26 g, 0.0029 mol) dissolved in THF was charged (transferring via cannula) at -78 oC. After the complete transfer of CoCl2 the reaction mixture was allowed to warm to room temperature and stirred for 3 h. Solvent was evaporated under vacuum and the dark green colour product was extracted with toluene until the extraction became colourless. The combined extracts were consolidated and evaporated to dryness. To this crude product, THF was added (20 mL) to dissolve and cooled to -78 oC. The precipitate was filtered and dried having a yield of 0.5 g (67%) of green microcrystalline product.
Elemental analysis, Calcd for C36H32Co2: C 73.22, H 6.77; found: C 73.16, H 6.57.
FTIR: (Nujol, NaCl disk) 560 cm-1 (corresponding to metal -carbon bonding).
Results and Discussion
Synthesis and characterisation of [(η5-L1)2Fe2] (1)
We have adopted the salt elimination method for the synthesis of bis-indacenyl complex. Making the dianion of the ligand L1 (Li+2L12-) with two moles of anhydrous FeCl2, that was prepared in situ, as shown in Figure 2 the reaction affords a red solution and after purification red micro crystalline product was obtained that was highly sensitive to air. The NMR spectrum shows that it has two isomers, depending on the position of the hydrogen of the indacenyl ring. No attempts were made to separate the isomers. Elemental analysis was in good agreement with the calculated value. FTIR show the formation of the characteristic metal-carbon bond frequency at 611 cm-1.
Synthesis and characterisation of [η5-L1) Fe(η5-L1)Co] (2)
This compound was prepared by stepwise salt elimination by treating ligand L1 with one equivalent of n-butyl lithium to produce monoanion of the ligand (Li+1L1-1) and this was first treated with one mole of anhydrous FeCl2producing the ferrocene type complex with L1 (Figure 3). The compound so produced was reported earlier by the Manriquez group. The characterisation of this compound matches well with the reported findings [18-19]. This mono iron organometallic complex was further treated with two mole equivalent of n-butyl lithium and with one equivalent of anhydrous CoCl2 affording the brown colour solution. After purification and drying, itoffered the brown coloured compound. The NMR shows the two shifts for the C-H of Cp rings in indacene at 4.92 and 5.25 ppm respectively for cobalt and iron next tothe bis indacenyl ring. However, due to residual paramagnetism, the peaks are broad. The IR frequency characteristic of the metal-carbon bond appeared at 585 cm-1.
Synthesis and characterisation of [η5-L2)2Co2](3)
We have synthesised 3 in like manner as the synthesis of 1 by taking anhydrousCoCl2and the reaction offered the dark green solution. After purification and drying it offered the off green coloured powder. Elemental analysis and FTIR confirm the formation of the compound. The IR absorptions corresponding to metal-carbon bonding appeared from 567 to 580 cm-1.
The salt elimination method for preparing these compounds gave a good yield. Attempts were made to partially oxidise these complexes with ferrocenium tetra fluor borate. Even after several hours of reflux, the isolated product shows similar spectral characteristics to that of complexes 1, 2, and 3suggesting that oxidation if any is only due to the ligand.
Electrochemical characterisation
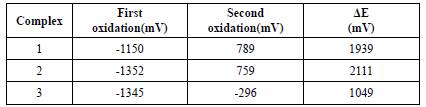
Cyclic voltammetry studies of complexes 1, 2, and 3were carried out in dichloromethane solvent with tetrabutyl ammonium tetra fluoroborate as a supporting electrolyte. Cyclic voltammogram of 1 presents a first reversible redox couple and a second irreversible couple. The potential values change with the scan rate. At 50 mVs-1. The oxidations (Epa) occur at Epa1 = -1150 and Epa2 = 0.789 vs the saturated calomel electrode. Controlled potential electrolysis at 0.789 V allowed us to determine that 1 equivalent of charge per mole of the complex has been transferred in this first redox process. Separation between two oxidation potentials AE is 1939 mV reveals the high degree of electronic delocalisation in the system. Complex 2also shows a much stronger interaction than complex 1 (Figure 4). At 50 mVs-1 scan rate in the dichloromethane as solvent, the first oxidation occurs at -1352 mV attributed to the oxidation of Co+2 to Co+3 and the second oxidation at 759 mV corresponds to the oxidation of Fe+2 to Fe+3. AE between two oxidation potentials is 2111 mV. This suggests the strong coupling between the metal and the ligand.
The complexes do not show any electrochemical reversibility. The results are summarised on Table 1. This value of ΔE indicates the strong interactions between the metal centres, indicative of Class III complexes according to the modern method of classification and the Robin and Day Classification [20-21].
Mossbauer spectroscopic characterisation of complex 1 and 3
Mossbauer spectroscopy is a local probe technique that is very sensitive to the oxidation state and the crystallographic environment of the iron. Quadruple splitting and hyperfine interaction parameters are summarised on Table 2. On Table 2, it is evident that the Iron is in +3 oxidation state for complexes 1 and 3 by observing that the FWHM value reveals the high spin Fe(III) of the complexes. At room temperature, thermal activation promotes the electrons to a close enough excited molecular orbital state and hence we observe the change in the oxidation state from Fe+2 to Fe+3. Similar behaviour was also observed in other reported complexes [22].
The possible change in the oxidation state is further evidenced by the DFT calculations. The calculations were carried out on the similar compound L2Fe2 where L = 2,6 di phenyl 4,8 di methyl s-indacene. This indicates there exists a charge transfer from one metal centre to the other. In Figure 5, the colour red shows the depletion in the electron density and the colour blue shows the increase in electron density. The molecular orbital diagram in Figure 6 shows the ground state isa triplet.
Magnetic measurements of complexes 1, 2 and 3
Field cooled magnetisation and zero field cooled magnetisation data were taken for all the complexes. Tc was estimated from the extrapolation of the linear portion of the M(T) curve. The M(T) plots decrease rapidly as the sample warmed from 4K. The sharp transition at Tc suggests some degree of structural and magnetic uniformity throughout the complexes. The Tc's of the different complexes range from 25 to 40 K with high reproducibility. The overlap of FC and ZFC suggests that there is no magnetic anisotropy in complexes 1 and 3, whereas for complex 2 there is a bifurcation of the FC and ZFC plots at 32K suggesting the possible phase transition in this complex as shown in Figure 7.
Measurements of magnetisation versus applied field, M(H) were carried out on each material at 3 and 300 K. The results are summarised on Table 3 and the representative sample is plotted in Figure 8. It is interesting to observe that there is a large difference in the coercivity values from 3 to 300 K. The decrease in the coercivity value at higher temperature suggests that the complexes tend to reach a super paramagnetic at higher temperature
The reciprocal of magnetic susceptibility obeys the Curie-Weiss law in the temperature range from 20 to 300 K. The Weiss constant was obtained by way of the Curie-Weiss law. The 0 value was -4.566, indicating that there exists a non-negligible antiferromagnetic coupling in this complex.
Conclusions
We have successfully synthesised metal complexes with substituted s-indacene ligand by means of salt elimination. Mossbauer study shows the oxidised metal centres. Furthermore, the attempts of partial oxidisation of metal centres in the complexes either chemically or electrochemically failed suggesting the ligand centred oxidation. The magnetic measurements suggest that the complexes synthesised are magnetic at lower temperatures suggesting the spin cross over phenomenon. Electrochemical characterisation suggests the strong electronic delocalisation in these complexes. A future research goal in this area is to discern the suitability of these complexes for magnetic applications in memory storage devices. We are trying to encapsulate the complexes to avoid air sensitivity for said applications.