Introduction
The Cape gooseberry is a highly exported exotic fruit in Colombia (Peña, Ayala, Fischer, Chávez, Cárdenas-Hernández, & Almanza, 2010). According to Legiscomex (2013), in 2011, 743 ha were planted, of which, 52.2% were in Boyacá, 20.7% were in Antioquia and 10.1% were in Cundinamarca, for a total production of 10.771 tons.
Cape gooseberry fruits (Physalis peruviana L.) are highly desired because of their high mineral content, flavor, and functional and medicinal properties (Balaguera-López, Martínez, & Herrera-Arévalo, 2014). This fruit has a climacteric behavior, with changes in color, aroma, and firmness during ripening and increases the soluble sugars content and antioxidant activity, processes that continue rapidly under natural conditions, making it a highly perishable fruit (Fischer, Almanza-Merchán, & Miranda, 2014).
Calcium is important for preserving the quality of fruits and vegetables because it contributes to the reduction and control of many disorders in fruits, mainly through the stabilization and rigidity of cell membranes and walls (Amézquita, Balaguera-López, & Álvarez-Herrera, 2008). An increase in calcium in the cell walls of fruits can delay softening by forming ionic bridges among calcium and free carboxylic groups of galacturonic acid residues which are present in pectins and strengthen the cell wall structure (Angeletti, Castagnasso, Miceli, Terminiello, Concellón, Chaves, & Vicente, 2010). Likewise, calcium treatments have been shown to be effective in some cases at reducing mold growth, reducing the incidence of physiological disorders and affecting respiration (Ramírez, Galvis, & Fischer, 2005; Hernández-Muños, Almenar, Ocio, & Gavara, 2006).
Different studies have shown immersions in calcium increase the content of this element in the cell wall of fruits, slow respiration, reduce ethylene production and increase the shelf-life of fruits such as strawberry, apple, papaya and avocado (Figueroa, Opazo, Vera, Arraigada, Díaz, & Moya-León, 2012), along with an increase in the postharvest life. Amézquita et al., (2008), evaluated the effect of preharvest foliar applications of calcium and gibberellin on Cape gooseberry fruits and observed less fruit cracking.
Therefore, the aim of this research was to determine the effect of different concentrations of calcium chloride and proper refrigeration on the postharvest behavior and organoleptic characteristics of the Cape gooseberry in the municipality of Ventaquemada, Boyacá-Colombia.
Material and methods
For this research, Cape gooseberry fruits, ecotype Colombia, harvested from a commercial crop in the municipality of Ventaquemada (Boyacá, Colombia), located at 2630 meters above sea level (m. a. s. l.) with an average temperature of 14°C, were used. The fruits were taken to the plant physiology laboratory of the Agronomical engineering program at the Universidad Pedagógica y Tecnológica de Colombia - Tunja, Boyaca, Colombia to carry out the present research and determine the required measurements.
Experimental design
A completely randomized design with four treatments was performed: the first corresponded to fruits with an application of 0.5% CaCl2, the second, an application of 1% CaCl2, the third, corresponded to fruits with an application of 2% CaCl2, and the fourth, was the control. Containing only fruits with the calyx. Each treatment was repeated four times, for a total of 16 experimental units (EU). Each EU consisted of approximately 140 g of fruit. The fruits were collected in stage 6, according to Icontec NTC 4580 (1999), that is, completely healthy with a homogeneous fruit size. The fruits were kept at a temperature of 21 ± 1°C and 45% relative humidity, respectively.
At 7, 14, 21, 28 and 35 days after harvest (DAH), measurements of the color index (CI) were taken, calculated with the CIELab parameters L, a, and b using a digital colorimeter, Minolta CR300 (Minolta Co., Japan); along with fruit firmness (N) using a PCE-PTR200 (PCE-Iberica, Spain) digital penetrometer. The mass loss (%) was determined with an electronic scale, 0.01g Precision, Acculab VIC 612 (Sartorius Group, Germany). The total soluble solids (TSS) were measured with a HANNA HI 96803 (Hanna Instruments, Spain) digital refractometer, with a 0-85% range and 0.1° Brix accuracy. The total titratable acids (TTA) were determined with calculations with the NaOH volume data, incorporated in 5 g of fruit juice, adding 3 drops of phenolphthalein in a potentiometric titration, to a pH of 8.2. For the pH, 2 ml of Cape gooseberry juice were used and a reading was determined using a HANNA HI8424 potentiometer (Hanna Instruments, Spain).
Statistical analysis
The data were performed for tests of normality and variance homogeneity. Afterwards, an analysis of variance was carried out to determine the statistical differences. In order to determine the better treatments, Duncan tests were carried out (P≤0.05). SAS v. 9.2 and used for the analyses (SAS Institute Inc., Cary, NC).
Results and discussion
Mass loss
In all of the treatments, the mass loss increased over time (Figure 1).
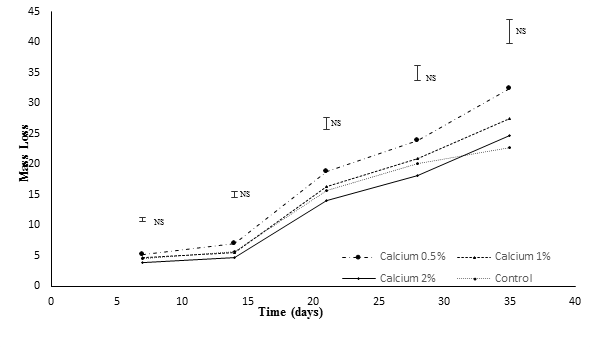
Figure 1 Effect of calcium applications on mass loss in fruits of Cape gooseberry. * Significant difference with the Duncan test (P≤0.05); NS: no significant. Error bars indicate standard error.
The loss percentage decreased in the fruits as the concentration of CaCl2 increased. The control fruits had the biggest loss, while the fruits in the CaCl2 treatments saw lower values of mass loss, which agrees with the findings of Galvis, Arjona, & Fischer (2003), who observed mango fruits (Mangifera indica L.), variety Van Dyke, performed a decreased mass loss rate with increased concentrations of CaCl2 in the treatments and the control fruits had the biggest loss. On the other hand, the fruits subjected to the treatment with 2% CaCl2, performed the lowest loss. Shirzadeh, Rabiei, & Sharafi (2011), reported that apples dipped in solutions with different Ca concentrations presented a lower mass loss, as compared to the control. The maximum mass loss in the Cape gooseberry fruits was in the control treatment, while the lowest loss was recorded in the 2% CaCl2 application.
Mahajan & Dhatt (2004), stated pear fruits treated with CaCl2 performed a reduced mass loss, as compared to the untreated fruits, during a storage period of 75 days. The results may differ due to differences in the immersion time and concentrations of CaCl2, which facilitated a greater or lesser degree of calcium entry into the fruits, which, according to Saure (2005), depends on factors such as the degree of maturity, the fruit structure, the dose and the immersion time in the solution.
Firmness
The firmness decreased continuously during the assessment period, consistent with the report by Amézquita et al., (2008) and Balaguera-Lopez et al., (2014). There were only significantly differences at 30 dah, where the greatest firmness was obtained in the fruits treated with 1% CaCl2. The control performed the lowest value after the last postharvest measurement, similar to the report by Amézquita et al., (2008), when subjecting Cape gooseberry fruits to 0.5% CaCl2, the firmness presented a longest period of time respect to other treatments.
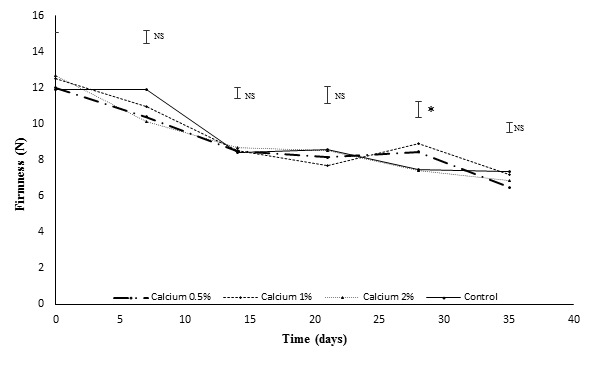
Figure 2 Effect of calcium applications on firmness in fruits of Cape gooseberry. *Significantly difference with the Duncan test (P≤0.05); NS: no significant. Error bars indicate standard error.
For the reduction in fruit softening, the exogenous calcium applications were effective due they have allowed to have a stimulatory effect on the synthesis of various cell wall components, which causes a slowly degradation, which may help to maintain the integrity of the cell wall and extend fruit quality during storage (Galvis et al., 2003). Calcium binds to the negatively charged residues of esterified uronic acids generated by the enzyme pectin methyl esterase during ripening, which increases the mechanical strength of the tissues.
Superficial color
The increase in chromaticity, from green to red, (a) during the postharvest period may be due to the gain in the dark orange color in cape gooseberry fruits due to oxidation processes that occur during maturation, which lead to the degradation of chlorophylls, caused by chlorophyllase, peroxidase and pheophytins (Cogo, Chaves, Schirmer, Zambiazi, Nora, Silva, & Rombaldi, 2011). The b value of the fruits decreased during storage, attributed to the instability of the carotenoids, which have a highly unsaturated chemical structure that favors oxidation processes (Luchese, Gurak, & Ferreira, 2015).
The value of the color index (CI) increased during the storage of the fruits, without significantly differences. Color changes in Cape gooseberry fruits are due to chlorophyll degradation, accumulation of carotenoids and exposure of pigments formed during maturation (Ferrer, Remón, Negueruela, & Oria, 2005). The results agreed with the findings of Ernani, Dias, Amarante, Ribeiro, & Rogeri. (2008), who reported applications of 0.5% CaCl2 at different frequencies (0, 4, 8, 12 times.year-1) on apple trees, did not present significantly differences in the fruit quality in terms of firmness, TSS, TTA, starch content and color.
Total soluble solids (TSS)
The treatments performed significantly differences respect to the control. The application of 0.5% CaCl2 exhibited and average values of 16.32 and was the treatment with the highest sugar content, in agreement with Gastelum-Osorio, Sandoval-Villa, Trejo-López & Castro-Brindis (2013). According to Menéndez, Lozano, Arenas, Bermúdez, Martínez, & Jiménez (2006), an increase in TSS occurs due to the starch hydrolysis and polysaccharides of the cell wall, giving rise to soluble sugars (Figure 4).
According to Hernández-Muñoz et al., (2006), this is due to water loss through transpiration that is greater than the loss of carbohydrates produced by respiration.
The fruits without any application of CaCl2 performed a decline among days 28 and 35 of the postharvest storage. This behavior could be related to the increased respiration at room temperature during the postharvest period, increasing the consumption of sugars in some processes, such as glycolysis (Sharma, Krishna, Patel, Dahuja, & Singh, 2006).
Total titratable acidity (TTA)
The acidity increased over time, from the beginning of storage and up to day 14, showing a significantly difference. In addition, it decreased until the last day of the postharvest period, resulting from the progress of the maturation process due to organic acids are used as substrates in respiration or are converted into sugars throughout gluconeogenesis, meaning maturation leads to a fall in acidity (Lanchero, Velandia, Fischer, Varela, & García, 2007). This result agrees with the findings of Amézquita et al., (2008), who reported the application of calcium, favors the postharvest life of Cape gooseberry fruits due to a higher acid content at harvest time which results in a longer shelf-life.
pH
The treatments performed significantly differences. The highest value was obtained with the application of 0.5% CaCl2, while the lowest value was 1% CaCl2. A decrease in the pH value was observed throughout the postharvest period, indicating the degradation of some acids (Marschner, 2002). This behavior possibly occurs due to higher concentrations of calcium in fruits, enable a reduction of the polygalactunorase activity and, therefore, smaller increases in the pH levels (Marschner, 2002). With storage at room temperature, the pH values increased during the experiment, which, according to Ramirez et al., (2005), was due to the higher temperature, which increases respiration rates, and other metabolic processes will be greater than in fruits stored at low temperatures.
Conclusion
Under the experimental conditions, the application of calcium on the cape gooseberry fruits decreased the mass loss, firmness and TTA. The fruits which received calcium applications performed an increase in the TSS and CI in the postharvest period. The doses used in this research delayed ripening and promoted the preservation of fruit quality for a longer period


















