Introduction
The color is the first quality control for consumers, delivering a notion of sweetness, flavor, salinity and allowing differentiate decomposed products (Abdel-Aal et al., 2014; Li et al., 2015; Wadhera and Capalde-Phillips. 2013). Therefore, the food industry has used artificial colors, enhancing the natural colors of food; however, they have presented adverse effects such as inhibition of immune system, allergic reactions, attention deficit hyperactivity disorder cases, asthma, abdominal pain, damage to kidney and liver and have been related to some types of cancer (He and Giusti, 2010; Jampani and Raghavarao, 2015;).
On the other hand, natural colorants that are environmentally friendly, lack toxicity and possess a variety of beneficial properties; calling the attention of the industry and consumers, producing an increase in the study of this type of pigments (Cortés et al., 2017; Zou and Liu, 2014). Anthocyanins are a group with more than 635 compounds, soluble in water, which are commonly responsible for purple, blue and red colors of flowers, fruits and plants, providing protection for biotic and abiotic stress (He and Giusti. 2010; He et al., 2016; Syafinar et al., 2015).
It has been reported that purple cabbage (Brassica olerácea L.) contains anthocyanins, being cyanidin 3-sophoroside-5-glucoside and cyanidin 3-sophoroside-5-glucoside acetylated with synapic acid, ferulic acid, p-coumaric acid and malonic acid (Bernstein and Zapata, 2015a; Hongmei and Meng, 2015. Anthocyanins have beneficial properties such as the prevention of obesity and providing protection against cardiovascular diseases, diabetes, cancer; in addition, it has been described that high concentrations of this natural pigment have not presented toxic, teratogenic or mutagenic effects (He and Giusti, 2010; He et al., 2016a; He et al., 2016b; Zou and Liu, 2014;). The purple cabbage has a favorable influence on human health (Bernstein and Zapata, 2015b). Currently, red cabbage pigment is being used in beverages, candies, dry mixes, chewing gum, a variety of sauces, and yogurt (Chigurupati et al., 2002).
Among the extraction methods that have been used to obtain the anthocyanin bioactive compound is microwave extraction, known to be an environmentally friendly method, which is based on the direct impact on polar compounds through heat and pressure that this technique exerts on the matrix that contains the compound of interest (Barba et al., 2016).
It has been reported that the extraction of bioactive compounds through the microwave technique at low intensities (24 min at 180 W) and medium (8 min at 540 W) the myrosinase enzyme does not substantially lose its activity; however, with a high power (4.8 min at 900 W) the total loss of the hydrolytic activity of the enzyme is obtained. Therefore, it can be established that the temperature profiles obtained after a microwave treatment play a fundamental role in the extraction of bioactive compounds, since in the case of the Brassica species with a higher retention of glucosinates and a controlled activity of myrosinase may offer an increase in its health promotion properties (Verkerk and Dekker, 2004).
Several studies have established that anthocyanins are highly unstable, being affected by light, pH, SO*, storage temperature among others (Hongmei and Meng, 2015; Zaidel et al., 2014); consequently, microencapsulation has been implemented as an efficient method to protect the natural compound from adverse environmental conditions, thus increasing its half-life (Mahdavi et al., 2016).
The objective of this study was to determine the best anthocyanin extraction condition present in the purple cabbage by microwaves with short times, in addition to perform the microencapsulation and determine the stability of the anthocyanins in a fermented milk beverage for a short storage period.
Materials and methods Materials
The purple cabbage (Brassica olerácea L. ssp Capitata f. Rubra), the fermented milk beverage with coloration similar to white (pineapple flavor) and the market sample (fermented milky beverage, blackberry flavor) were obtained in a supermarket in Antofagasta city (Chile). Meanwhile, ethyl alcohol grade P.A. and potassium chloride were purchased from the company Winkler (Santiago, Chile). Citric acid was purchased from Analytic (Santiago, Chile). Sodium acetate trihydrate and hydrochloric acid were purchased from the company Sigma-Aldrich (Darmstadt, Germany). Regarding the wall materials used, inulin was donated by the company Mieles de Campos Azules S.A de C.V. (Guadalajara, Mexico); whereas, maltodextrin (DE = 12) and gum arabic were donated by the company Blumos (Santiago, Chile).
Preparation of sample
Fresh purple cabbage leaves were selected and weighed on a GX-1500 Great Precision model scale (Shimadzu, Japan). Then, leaves were subjected to the bleaching process for enzymatic inactivation, exposing them to steam for 4 minutes (100 °C), immediately afterwards they were extracted and submerged in water at room temperature (20.0 ± 3.0 °C), causing a rapid decrease in temperature. The leaves were then drained and placed in a freezing chamber model QB0.4L2 (LXS, China) at a temperature of -18 ± 2 °C until they reached room temperature.
The grinding of the leaves was carried out immediately in a vegetable chopper model 1.2.3 (Moulinex, France) and kept at 4 ± 2 °C in a domestic refrigerator model FR-385S (Daewoo, South Korea) protected from light with aluminum foil until its use.
Color determination of fresh and treated leaves was determined through CIEL*a*b* system with the Colorflex color model 45°/0° (Hunterlab, USA) with daylight (10°/D65) and a port size of 0.75 inches, calibrating with the black and white tile provided by the equipment. The parameters L*, a* and b* indícate the trend towards white/ black, red/green, and blue/yellow, respectively. For each sample, at least 10 measurements of sample were made and each of the samples was determined in triplicate. For each sample, at least 10 measurements were made at different positions of the leaves and each of the samples was determined in triplicate.
Extraction Solutions
Two extraction solutions were prepared according to Arrazola, et al. (2014) with some modifications. The first contained a mixture of water with ethyl alcohol in the same ratio (1:1) and the second containing only water; additionally, two variants were presented regarding the acidification of these solutions through the addition of citric acid; being an acidified variety at 2% and another at 4%; so: -Solution 1.A = water plus ethyl alcohol 2% acidified, -Solution 1.B = water plus ethyl alcohol 4% acidified, -Solution 2.A = water 2% acidified, -Solution 2.B = water 4% acidified.
Anthocyanin extraction
For all extractions was used the microwave model MF-28G (Fensa, China), reaching a power of 1100 w. First 20 g of purple cabbage leaves blanched were mixed with 50 mL of water and ethyl alcohol 2% acidified with citric acid and exposed to two potencies (550 and 880 W) and two different times (60 and 90 s). Microwave parameters with which better performance was obtained were used for each of the four different solutions in the extraction process, using 200 g of purple cabbage leaves blanched, and 500 mL of solution.
After application of the microwaves all samples were filtered under vacuum with a filter paper (0.45 pm) using a kitasato flask with the help of the vacuum pump model V-100 (Buchi, Switzerland). Then, samples were centrifuged at 5000 rpm for 10 minutes in a Champion S-50D centrifuge (Ample Scientific, USA); recovering the supernatant.
Determination of anthocyanins contents
The determination of anthocyanins in the extraction solution was measured using the spectrophotometric method by differential pH (Xu et al. 2010). A solution of potassium chloride (0.025 M) was prepared at pH 1.0 and a solution of sodium acetate (0.4 M) at pH 4.5; adjusting the pH with hydrochloric acid, using the pH meter model Mi 151 (Martini Instruments, USA).
Absorbance of the samples was determined at 530 and 770 nm in the UV-Visible spectrophotometer model UV-1280 (Shimadzu, Japan); the buffer solutions were used as targets and the content of total anthocyanins (CAT) was calculated according to the eq. (1), established by Lee et al. (2005).
where: AbS is [A530 -pH L0) - A700 (pH h°)] - [A530 (pH 4.5) - A700 (pH 4.5)]; MW is the molecular weight of anthocyanin for cyanidin-3-glucoside (449.2 g/mol); DF is the dilution factor; is the extinction coefficient (26 900 L/cm*mol) and is the length of the optical path (1 cm).
Microencapsulation preparation
Total solids (ST) of the anthocyanins extracted in solution were determined with an infrared scale model ML-50 moisture analyzer (A&D Weighing, Japan), if ST <7% the sample was concentrated in a rotaevaporator model R-300 (Buchi, Switzerland) at 50 °C and 15 rpm of rotation, until obtaining a percentage of ST > 7%.
Wall materials (WM) of the maltodextrin (MD), inulin (IN) and gum arabic (GA) type were used in a ratio of MP to anthocyanin extract of 2:1.
WM were mixed with anthocyanin extract without prior hydration, according to the established ratio, using a blade stirrer model RW Basic (IKA, Germany) at 735 rpm for 15 minutes, then the mixture was exposed at 35 000 rpm for 15 minutes in an ultraturrax model Pro-200 (Proscientific, USA). Finally, the CP130 ultrasound apparatus (Cole Parmer, USA) was used to homogenize the mixture for 10 minutes, with an amplitude of 80 s and without oscillations.
Two different values for the inlet air temperature were applied to the spray dryer model Mini Spray Dryer B-290 (Buchi, Switzerland) being 150 and 170 °C, with a suction pump flow of 35 to 38 m3/h and the flow of the feed pump was maintained at 25 mL/min. The yields of each microencapsule obtained were calculated with the eq. (2), in addition to the chromatic coordinates.
where, REN is the performance of the microencapsulation in mass percentage; W m is the total weight obtained from the microencapsulation (g); and W o is the weight of the ST of the inlet solution to the dryer (g).
Stability analysis
Anthocyanin microencapsulation with the highest yield was added to a fermented milk drink with a color similar to white, to imitate the color of a commercially available purple milk beverage called commercial sample (CS).
Four formulations with different amounts of microencapsulation (0.20, 0.25, 0.30, 0.40 g) were made in 25 mL of milk fermented beverage and the chromatic coordinates were measured through the CIEL*a*b* system, as described previously but using a port size of 1.25 inches
Then with the eq. (3) color difference (AE) was determined, being calculated in relation to the market sample.
where,
ΔL = L*2 - L*1: Luminosity difference, Δa = a*2 - a*1 = Red - green difference, Δb = b*2 - b*1 = Blue - yellow difference.
The subscripts 1 and 2 serve to designate two moments of the measurement (initial and final) between which the color difference is calculated.
The formulation with the least variation of AE was selected to perform the stability analysis. For this, 6 g of anthocyanin microencapsulation were added to 500 mL of fermented milk drink and then in 24 bottles of 20 mL of clear glass and twist off caps were filled with pigmented fermented milk drink and stored in darkness at 4 °C. This study was performed for a short storage period refrigerated for 8 days, the color was measured in triplicate and the AE was calculated in relation to day 0.
Results and discussion
Anthocyanin extraction from red cabbage
In Table 1 chromatic coordinates obtained for each stage of the anthocyanin extraction process are presented; establishing that the microencapsulation was the one with the highest luminosity value ( L* = 63.39 ± 0.07); while, the concentrate showed a strong tendency towards red (a* = 60.51 ± 0.04), additionally it was the only stage in the process that presented a positive value for the chromatic coordinate b* (17.18 ± 0.08). For the coordinates L* and a* all the stages had statistically significant differences (P < 0.05) between them; however, regarding the chromatic coordinate b*, there were no significant differences between complete and crushed leaves of purple cabbage, despite there being significant differences in the coordinates for the other two stages (concentrated and microencapsulated).
Table 1 Chromatic coordinates for each stage of the process carried out with the purple cabbage: scaling, extraction and microencapsulation.

Different letters in the same column indicate significant difference (p < 0.05) analyzed by Duncan’s multiple range test.
The highest CAT obtained with a solution of water plus ethyl alcohol 2% acidified was 149.29 ± 1.66 mg/L for a power of 880 W during 90 s; however, the second largest CAT was obtained with a power of 550 W and a time of 90 s (Figure 1). The most important influence is the time of application of the microwaves, since at a longer time a considerable concentration of anthocyanins was obtained. In another research work with anthocyanin extracts from purple cabbage, it was determined that the CAT decreases from 13.68 to 6.48 mg/100 g in anaerobic conditions and from 13.77 to 5.01 mg/100g in aerobic conditions, to the extent that the pH increases from 3.0 to 5.0 for increases in acidity, achieved with ascorbic acid in concentrations up to 200 mg/100 g (Walkowiak-Tomczak and Czapski. 2007). Work has been carried out where one of the variables of extraction is time, as it is in the case of obtaining anthocyanins from blueberries, where the highest extraction was at 5.3 minutes, decreasing significantly from this value due to the heat generated by the microwave equipment that helps the degradation of this type of bioactive compounds (Zheng et al., 2013).

Figure 1 Concentration of total anthocyanins (CAT) through microwave extraction using different power and time parameters with a solution of extraction of water and ethyl alcohol acidified at 2% with citric acid.
Using the parameters that showed the highest extraction of anthocyanins, it can be established that for two extraction solutions with their two variants, the highest CAT was obtained with the solution of water plus ethyl alcohol in its 2% acidification variants giving a CAT of 199.00 ± 2.00 mg/L and 170.30 ± 1.20 mg/L for the acidification of 4% (Figure 2).
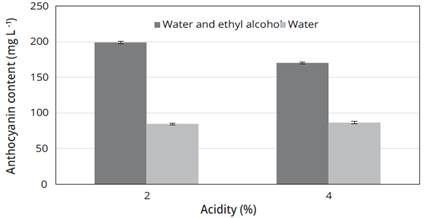
Figure 2 Microwave extraction of anthocyanins using different extraction Solutions, with an application time of 90 s and a power of 880 W.
The molecules belonging to the group of anthocyanins, can be dissolved in polar solvents, such as water and ethanol; however, they have a greater affinity to polar organic solvents, where ethanol, methanol or acetone are included (Zapata et al., 2014). This same behavior was observed in the extraction of anthocyanins from blueberries using microwaves and an ethanol solution (Zheng et al., 2013). On the other hand, this method has proven to be highly fast, since are 90 s; while in other works anthocyanin extractions have been made from purple cabbage that last 39.47 min, obtaining as a result 128.78 ± 2.24 mg /100 g (Zou and Liu. 2014). Nevertheless, when there is an extraction of anthocyanins by high pressure with CO2, the yield is very similar to that obtained by microwave, being 58.29 ± 0.56 mg/100 g (Xu et al., 2010).
Anthocyanin microencapsulation parameters
The best yields with respect to the initial solids content were obtained with the WM maltodextrin with 54 and 51% at dryer inlet temperatures of 150 and 170 °C, respectively. On the other hand, the lowest yields were 24.0 and 32.5% for input temperatures of 150 and 170 °C, respectively, both granted by the WM inulin (Table 2).
Table 2 Microencapsulation using as wall materials (WM) encapsulant maltodextrin (MD), inulin (IN) and gum arabic (GA) in a ratio of 2 parts of WM and 1 part of sample (2:1) and with a feed pump flow constant (25 mL/min).

* Total solids of the concentrated anthocyanin solution without the wall material.
Although both IN and MD are sugar derivatives (fructose and starch respectively), it has been shown that IN presents a high hygroscopicity (Fernandes et al., 2014), which is given because the molecule has several hydrogen bonds available that easily capture water molecules present in the environment, resulting in a loss of microencapsulation by adhering to the walls of the spray dryer (Flores-Belmont et al., 2015). On the other hand, it should be noted that the anthocyanins microencapsulated with inulin have been described as having greater stability than when maltodextrin is used (Robert and Fredes, 2015).
When MD presenting a higher yield, was evaluated at three different entrance temperatures (150, 160 and 170 °C) and in two proportions, being 2:1 and 1:1 of MD: anthocyanin concentrate. As can be seen, Table 3 shows the yields of the drying process, where it can be established that the best yield was obtained at an inlet temperature of 150 °C with yield of 58.9% and with the formulation 2:1 (MD: anthocyanin concentrate), followed by a yield of 57.0% for the same entry temperature, but with a different ratio of WM (1:1).
Table 3 Microencapsulation parameters for various inlet air temperatures with different ratio of wall material (maltodextrin):total solids using a constant feed pump flow (25 mL/min).

*WM:TS.- Wall material:Total solids of the concentrated anthocyanin solution.
It has been established in previous works that the air inlet temperature in the dryer has an impact on the obtained yield; standing out that at temperatures >160 °C the yields decrease (Ersus and Yurdagel, 2007).
Stability analysis of pigmented fermented milk beverage
The microencapsulation of anthocyanin with the WM maltodextrin that presented a yield of 58.9% was added to the fermented milk matrix with a color similar to white, with various concentrations of microencapsulation ranging from 0.20 to 0.40 g in 25 mL of milk beverage. Table 4 shows the chromatic coordinates for each addition of microencapsulation to the fermented milk beverage and the color differences (AE) that were presented with respect to the commercial pattern; observing that the formulation that has 0.30 g of microencapsulation in 25 mL of fermented milk matrix was the one that presented the least variation of color (AE) with the commercial pattern, with a value of 3.57.
Table 4 Chromatic coordinates in the different formulations and color differences (AE) of the microencapsulated addition in the fermented milk beverage in relation to the market sample.

ANT = Microencapsulated added. MS = Market sample.
Different letters in the same column indicate significant difference (P < 0.05) analyzed by Duncan's multiple range test.
Table 5 shows the color measurements using the CIEL*a*b* system with their respective standard deviations. As for color variations presented by the pigmented fermented milk matrix during the 8 days that the stability analysis lasted with respect to day 0, it can be established that AE was increasing with time, being lowest value of 1.93 up to a value of 3.41; therefore, it can be established that during the evaluation period of the samples, they did not present a difference that was perceptible to the human eye, since it is established that the difference is visible when the value of AE > a 5.0 (Obón et al., 2009). On the other hand; there were no significant differences (P > 0.05) between day 2 and 6 as well as between day 4 and 8 for the chromatic coordinate L*; while the chromatic coordínate a* did not present statistically significant differences (P > 0.05) between days 2 and 4, as well as on days 6 and 8 the chromatic coordinate b* instead, presented statistically significant differences (P < 0.05) between the measured days.
Table 5 Chromatic coordinates of the pigmented fermented milk beverage with microencapsulated anthocyanin for 8 days and AE calculated based on the color difference presented by the sample with respect to time 0.

Different letters in the same column indicate significant difference (p<0.05) analyzed by Duncan's multiple range test.
Within the obtained colorimetric variation, it can be determined that the value of the chromatic coordinate a* decreases with time; while the chromatic coordinate L* and b* increase their value over time; therefore, it is expected that the samples will lean toward a yellow hue with the passing of days. From here, it follows that the anthocyanins present in the fermented milk beverage may be degrading slowly in chalcones, which are characterized by being colorless or pale yellow-colored chemical compounds (Arrazola et al., 2014; Castañeda-Ovando et al., 2009).
Conclusions
The best extraction parameters using microwave technology were: power 880 W at 90 s, using the water and ethyl alcohol with 2% acidified, obtaining the highest CAT in solution; which indicates that this technique is an efficient and fast way for the extraction of anthocyanins. The highest yield for spray drying was 58.9% with a drying air inlet and outlet temperature of 150 °C and 90 °C, respectively; and a 2:1 ratio (MD: anthocyanin concentrate).
When adding 0.3 g of microencapsulation in 25 mL of fermented milk drink, the color obtained was similar to the commercial one; on the other hand, between 0 and 8 days the AE was less than 5.0, thus is not perceptible for the human eye.

















