Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Acta Biológica Colombiana
Print version ISSN 0120-548X
Acta biol.Colomb. vol.19 no.3 Bogotá Sept./Dec. 2014
https://doi.org/10.15446/abc.v19n3.42457
Artículo de investigación
http://dx.doi.org/10.15446/abc.v19n3.42457
NESTING ECOLOGY OF THE OLIVE RIDLEY SEA TURTLE (Lepidochelys olivacea) (CHELONIIDAE) AT EL VALLE BEACH, NORTHERN PACIFIC, COLOMBIA
Ecología de anidación de la tortuga golfina (Lepidochelys olivacea) (Cheloniidae) en la Playa El Valle, Pacífico Norte, Colombia
KARLA G. BARRIENTOS-MUÑOZ1; M. Sc. (student); CRISTIAN RAMÍREZ-GALLEGO, M. Sc. (student); VIVIAN PÁEZ2, Ph. D.
1 Departamento de Biología, Universidad de Puerto Rico, Rio Piedras, San Juan, Puerto Rico-USA. biokeroz@gmail.com; ramirezgallego.cristian@gmail.com
2 Grupo Herpetológico Instituto de Biología, Universidad de Antioquia. Medellín, Colombia. vivianpaez1@gmail.com
Author for correspondence: Karla Barrientos, biokeroz@gmail.com
Received March 5th 2014, first decision May 8th 2014, accepted June 19th 2014.
Citation / Citar este artículo como: BARRIENTOS-MUÑOZ KG, RAMÍREZ-GALLEGO C, PÁEZ V. Nesting ecology of the olive ridley sea turtle (Lepidochelys olivacea) (Cheloniidae) at El Valle Beach, Northern Pacific, Colombia. Acta biol. Colomb. 2014;19(3):437-445.
ABSTRACT
The olive ridley (Lepidochelys olivacea) is the most common sea turtle to nest in Colombia. El Valle beach is considered the most important nesting beach for this species in South America. Intensive direct capture of nesting females and egg poaching for consumption and local commercial purposes has been a common practice for years. We conducted an analysis of the nesting ecology of the olive ridley on El Valle beach in the northern Pacific of Colombia in 2008. A total of 164 clutches were transferred to an artificial hatchery for protection. The peak of nesting occurred from the second half of August until the end of September, accounting for 64.6 % of all nests. Along the beach, the section most frequently used was Section 3, with 26 % of the nests. The nests were laid mainly in zone 3.69 %. We encountered 55 nesting females and marked 46 of them. Mean CCL was 64.9 ± 2.4 cm and mean CCW was 68.6 ± 2.6 cm. Females laid on average of 87.3 ± 14.2 eggs per clutch. We recorded two nesting events per female, with a mean inter-nesting period of 18.8 ± 4.2 days. The reproductive output for the season was 181.5 ± 34.8 eggs / female. Mean hatching success was 81.1 ± 12.1 % and mean emergence success was 77.6 ± 12.7 %. The incubation period was 65 ± 4.7 days. Our study is a valuable contribution to knowledge of the reproductive ecology of the olive ridley population regionally and globally.
Keywords: conservation, Eastern Pacific Ocean, emergence success, hatching success, turtle rookery.
RESUMEN
En Colombia, la tortuga golfina (Lepidochelys olivacea) es la más común de las tortugas anidantes. La playa El Valle es considerada la más importante para anidación en Suramérica. La intensa captura de hembras y saqueo de huevos para consumo y comercio local ha sido una práctica común por años. Realizamos un análisis de la ecología de anidación de la tortuga golfina en la Playa El Valle, Pacifico Norte, durante la temporada de 2008. Un total de 164 nidadas fueron transferidas a viveros de protección. El pico de anidación ocurrió durante la segunda quincena de agosto y septiembre con un 64,63 % de las posturas. A lo largo de la playa, el sector 3 presento la mayor frecuencia de anidación con 26 %. Los nidos fueron desovados principalmente en la zona 3, con 69 %. Encontramos 55 hembras y 46 fueron marcadas. El LCC fue (promedio± DE: 64,9 ± 2,4 cm) y ACC fue (promedio ± DE: 68,6 ± 2,6 cm). Las hembras desovaron 87,3 ± 14,2 huevos por nidada. Registramos dos eventos de anidación por hembra, con un intervalo de (promedio ± DE: 18,8 ± 4,2 días). El producto reproductivo fue (promedio ± DE: 181,5 ± 34,8 huevos por hembra). El promedio en éxito de eclosión fue 81,1 ±12,1 % y de emergencia 77,6 ± 12,7 %. El período de incubación fue (promedio ± DE: 65 ± 4,7 días). Nuestros datos son una valiosa contribución al conocimiento de la ecología reproductiva de la población de tortugas golfina regional y globalmente.
Palabras clave: colonia anidante, conservación, éxito de eclosión, éxito de emergencia, Océano Pacífico oriental.
INTRODUCTION
The olive ridley sea turtle (Lepidochelys olivacea) is considered the most abundant sea turtle species in the eastern Pacific Ocean (EPO), and probably in the world (Márquez, 1990; Pritchard, 1997; Marcovaldi, 2001). The species is distributed in tropical and subtropical waters worldwide. It is found regularly in the Indian and Pacific Oceans (Márquez, 1990; Pritchard and Plotkin, 1995) and also in the South Atlantic (Reichart, 1993; Fretey, 2001). Recently, Eguchi et al., (2007), using shipboard observations, estimated the at-sea population to number more than six million turtles in the EPO. At present, the species is categorized as vulnerable on the IUCN Red List (IUCN, 2013).
In the EPO rookery, the nesting distribution ranges from beaches in southern Mexico to Peru (Pritchard and Plotkin, 1995; Márquez et al., 1996; Spotila, 2004; Wester et al., 2011) and foraging individuals are found from California to Peru and Chile (Pritchard and Plotkin, 1995). Most of the EPO nesting sites support solitary nesters and receive small numbers of turtles, but in a few nesting beaches, including Mexico (La Escobilla and Morro Ayuta), Nicaragua (Playa Chacocente and Playa La Flor) and Costa Rica (Playa Ostional and Playa Nancite), the turtles nest in "arribadas", characterized by large numbers of females nesting synchronously on the beach (Pritchard and Plotkin, 1995; Márquez et al., 1996; Valverde and Gates, 1999; Chaves et al., 2005).
The nesting of the species in the southeastern Pacific is low-density compared to other EPO nesting sites. In Colombia, nesting has been reported as solitary (Ceballos-Fonseca et al., 2003) and as rare in Ecuador and Peru, which is the southern boundary for olive ridley nesting (Hays-Brown and Brown, 1982; Manrique et al., 2003; Álava et al., 2007; Kelez et al., 2009; Wester et al., 2011).
In the Pacific of Colombia, the olive ridley is the most commonly nesting sea turtle. The principal nesting sites (more than 100 turtles/beach/year) are El Valle, (northern Pacific) (Ceballos-Fonseca et al., 2003; Barrientos and Ramírez, 2008) and the Sanquianga Natural National Park, Amarales, Mulatos and Vigia (southern Pacific) (Amorocho et al., 1992). In addition, approximately 30 other beaches are used by olive ridleys for nesting in Colombia (Amorocho et al., 1992; Ceballos-Fonseca et al., 2003).
The El Valle beach is considered the most important nesting beach for olive ridleys in Colombia, and in South America (Martínez and Páez, 2000; Hinestroza and Páez, 2001; Barrientos and Ramírez, 2008). Monitoring of nesting female turtles occurs during the nesting season from July to December. The Fundación Natura (an NGO) and college students conducted systematic monitoring surveys from 1991 to 1999. Since 1991, a hatchery has been protecting nests and releasing hatchlings into the population. In 1990, this hatchery was initiated at the San Pichi Beach in the Utría Natural National Park but was transferred in 1991 to the El Valle beach. In the 1991 nesting season, 88 arrivals of olive ridley turtles were recorded on the beach, from which 56 females were marked, of which six renested. In 1992, 148 females were registered, and in 1993 30 other turtles were registered while nesting, and 47 nests were transferred. In 1994, there was an increase to 69 turtles registered on the beach, with 174 nests; in 1995, 46 turtles and 116 nests were recorded, and in 1996, 36 turtles and 128 nests were recorded. In 1998, 113 turtles were encountered on the beach and 91 nests were transferred to the hatchery facility, with a total of 8918 eggs. In the 1999, season there were 377 females recorded and 165 nests transferred (Martínez and Páez, 2000; Hinestroza and Páez, 2001).
Monitoring ceased in 1999, until 2008 when beach monitoring was resumed with the help of local researchers (Aspropaclu), the support of the community council of El Cedro, and the Fundación Natura, which hosted nightly patrols to tag nesting females and to transfer clutches to the hatchery. This was deemed necessary because intensive direct capture of nesting females and egg poaching for consumption and/or local sale by communities located close to the El Valle beach has been a common practice for years (Ceballos-Fonseca et al., 2003; Barrientos and Ramírez, 2008; Ramírez and Barrientos, 2012).
Here, we report an analysis of the nesting ecology of the olive ridleys on the El Valle beach, northern Colombian Pacific, during the entire 2008 nesting season. We estimated reproductive output, inter-nesting intervals, nesting and hatching success, and we describe the nesting process for this population. Finally, we assessed the importance of the El Valle beach as an olive ridley nesting site in the EPO, but the data are insufficient to establish population trends.
MATERIALS AND METHODS
Study site
The El Valle Beach (6°04'21, 00'N, 77°24'04, 62''W) is located on the northern Pacific coast of the Chocó region in Colombia (Fig. 1). The site is 8200-m-long (Barrientos and Ramírez, 2008), and a portion of the beach lies withing the Utría Natural National Park (PNNU) (Fig. 1). This area has been inhabited by Afro Colombian people for over 100 years. The beach is interrupted by three permanent water bodies: the El Valle river and two streams: La Cuevita and Coredó. It is a high-energy beach that is exposed and receives strong waves (Martínez and Páez, 2000). The tides are semidiurnal with a range that can exceed 4 m, forming offshore currents that can attain high speeds (close to 2 m / s) during times of high tide (Prahl et al., 1990).
Nesting activity
To determine spatial use by olive ridleys, the beach was divided into nine sections along its south to north axis. First eight sections of 1000-m and in sub-sectors of 200-m and a final section of 200-m long. We distinguished three horizontal zones from the water to the vegetation: Zone 1 (the inter-tidal zone, that lies below the high water mark), Zone 2 (the mid-zone, located above the high tide line) and Zone 3 (beginning where dune vegetation began and continuing to the back of the beach delimited by the presence of trees, grass, and creeping vegetation).
The El Valle beach was monitored both nightly and during morning surveys during the entire 2008 nesting season (July 20 to December 28). During this period, we patrolled the beach at night between 20:00 and 05:00, and all turtles encountered were tagged, measured and all nesting events were recorded. In the morning, between 6:00 and 9:00, we counted tracks to account for turtles that were missed the previous night and verified successful nesting events.
Nesting turtles
When we encountered a turtle, we recorded its activity as: emerging from the ocean, digging a body pit, digging an egg chamber, laying eggs, covering the nest, or returning to the water. We recorded the nesting status of each turtle encountered (or tracks seen when the turtle was missed) as (1) a false crawl if no nest was encountered, or (2) a nesting event if there was evidence the turtle had laid eggs.
After the turtles finished laying eggs, all four flippers were checked for external tags. If a tag was present, the tag number was recorded. But if the turtle was not tagged, we marked it with a two Inconel 625 metal tags on the right and left front flippers after egg laying had finished. We tagged all turtles following the methodology presented in the Research and Management Techniques for the Conservation of Sea Turtles (Eckert et al., 1999). For each turtle, we measured the curved carapace length (CCL) and curved carapace width (CCW) with a flexible measuring tape (± 0.05 cm). We counted eggs with a mechanical counter during egg laying, or at the time of relocation of the clutch to the hatchery for protection. We estimated the reproductive output for nesting season of each individual by multiplying the average number of eggs per clutch by the number of confirmed nests of each female.
Nest and hatcheries
We relocated all clutches to safer areas of the beach immediately after laying. In total, three hatchery areas were used for protecting the clutches during the incubation period, all of them located within the Estación Septiembre facility belonging to the Fundación Natura. We relocated eggs from nesting turtles we observed directly, or from other nests detected during the morning monitoring (less than six hours old), following standard protocols (see Chacón et al., 2007). Eggs were placed in plastic garbage bags (5 l) and reburied in hatchery sand.
After 45 days of incubation, we checked each nest eight times a day to inspect for evidence of hatchling emergence. Five days after the first signs of emergence were observed, a subset of the nests was excavated to determine hatching success. At excavation, we counted the number of shells and classified unhatched eggs into four developmental categories (Chacón et al., 2007). We estimated hatching success using the formula H (Hatching) = S / (S+U) * 100, where S is the number of empty shells encountered (>50 % complete), U is the number of unhatched eggs. We estimated emergence success using the formula E (Emergence) = S - (L+D) / (S+U) * 100, where L is the number of live hatchlings found in the nest, and D is the number of dead hatchlings that managed to first leave their egg shells (Miller, 1999). Hatchlings found alive were released in the evening in the company of members of the El Valle community.
Threats
Local threats to this species and other sea turtle species in the area were recorded.
Statistical analyses
All statistical analyses were conducted using the statistical software package R, version 3.0.1 (R Development Core Team, 2013). We analyzed normality of the CCL distributions with the Shapiro-Wilks test. We used ANOVA tests and linear regressions to analyze the relationships between female size variables (CCL and CCW) and to analyze the effect of female size on clutch size. Differences in the number of eggs among clutches laid by the same female were inspected with Paired t-tests.
RESULTS
Nesting activity
During the five months (July 20 to December 28, 2008), a total of 164 clutches were transferred to the hatchery, with a total of 176 successful nests laid and 202 female arrivals recorded. The peak of the nesting period ranged from the second half of August to the end of September (64.6 % of all nests, Fig. 2). The most active time for nesting was between 21:01- 22:00 hr.
The beach sections most frequently used were Sections 3 and 7, with 26 % and 20 % of nests laid, respectively (Fig. 3). We determined that over half of the nests were laid in Zone 3 (69 %), while in Zone 1 and 2,2 % and 30 % of all nests were laid, respectively (Fig. 3).
Nesting females and nests
At the El Valle beach, we encountered 55 nesting female olive ridleys and tagged 46 them with two Inconel metal tags on the front flippers after they had laid. The size of the females (CCL) was normally distributed during this season (W = 0.99, p = 0.79, Fig. 4). The CCL for turtles was (mean ± SD) 64.9 ± 2.4 cm (range 9.2 - 70.5 a 59.2 - 70.5 y n = a), and the CCW was 68.6 ± 2.6 cm (range 63.5 - 75.0 a 63.5 - 75.0 y n = a). In olive ridleys the typical pattern is for the carapace to be wider than is long. Therefore, for the following analysis we discarded one female with an atypical carapace: 66.8 cm CCL and 66.4 cm CCW. A strong correlation (r = 0.83) was found between the CCL and CCW (r2 = 0.681, ANOVA, F (1, 46 df) = 98.18; p < 0.001). The females laids on average 87.3 ± 14.2 yolked eggs per clutch (n = 46). We recorded a maximum of two nesting events per female, with an inter-nesting period of (mean ± SD) 18.8 ± 4.2 days (range 16 - 25 days; n = 4). These turtles tended to lay smaller clutch the second time, although the differences were not significant (t = 2.9 df = 6, p = 0.063).
The estimated reproductive output for the season was (mean ± SD) 181.5 ± 34.8 eggs per female (n = 4). Female CCL was correlated with clutch size (r2 = 0.101, ANOVA, F (1, 43 df) = 4.83, p = 0.03), but not significant relationship was found between CCW and clutch size (ANOVA, F (1, 43 df) = 0.21; p = 0.6457).
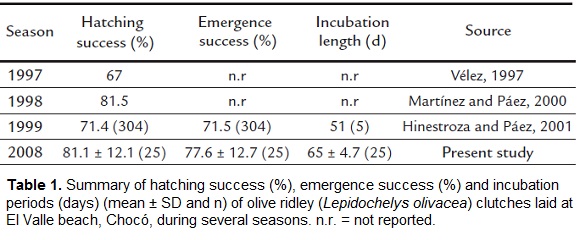
The mean hatching success from nests at the hatchery was 81.1 ± 12.1 % (range 45.8 - 100; n = 25) and mean emergence success was 77.6 ± 12.7 % (range 45.8 - 93.4; n = 25). The incubation period to emergence in the hatchery was (mean ± SD) 65 ± 4.7 days (range 54 - 73; n = 25).
Threats
We identified direct harvest of five olive ridley females and the poaching of 12 clutches by local people. This probably occurred early in the morning, between 4:00 - 6:00 hr, when patrol effort was lowest. We also recorded evidence of incidental by-catch in artisanal fisheries. Fishing gear such as long lines and gillnets are used in the waters off of the El Valle beach, a threat not only to the olive ridleys, but also for other species of sea turtles that feed there (e.g., the black turtle, Chelonia mydas and the hawksbill, Eretmochelys imbricata). During this project, one male olive ridley was harvested in the gill net of a local fisherman, who intended to consume it. However, project personnel alerted local authorities who confiscated and released this individual. Also a juvenile hawksbill was caught by in another gill net, but was returned voluntarily by its captor.
DISCUSSION
We confirmed for this olive ridley rookery a peak in nesting activity during the second half of August until the end of September, with over 64 % of all nests laid during this period (Fig. 2), as was also documented during the 1999 nesting season (Hinestroza and Páez, 2001). Peak time for nesting was between 21:00 and 22:00 hr, which also agrees with Hinestroza and Páez (2001), who reported a peak in the number of arrivals between 21:00 - 23:00 hr. Therefore, the early hours of the night appears to be the time when most turtles emerge to nest. However, this result also may have been due to a higher effort by project staff to monitor at the beginning of the night.
A total of 52 % of all L. olivacea nests were located in beach sections 3 (26 %) and 7 - 8 (26 %) (Fig. 3). Section 3 borders the Coredo stream in section 4 and sections 7 -8 border the El Valle river, indicating some preference for nesting near river mouths (Pritchard and Mortimer, 2000), where broader sand platforms are formed, producing better conditions for the nesting (Amorocho, 1993). In contrast, in 1998 and 1999 most nesting occurred in the three first sectors of the beach, but these also are located near the Cuevita stream (Martínez and Páez, 2000; Hinestroza and Páez, 2001).
Nesting female olive ridleys usually selected flat, open and unobstructed areas and deposit their clutches between the high tide line and the vegetational line (Cornelius, 1976; Reina et al., 2002). This behavior has been modified in some Colombian Pacific beaches, where females place their clutches between driftwood and the vegetation line, because the sand portion in this zone is almost completely inundated during high tides (Amorocho et al., 1992). In this study of the El Valle beach, females also preferred to nest in the highest beach area, on the dune (69 %). However, in the past females tended to nest most in the lowest (Martínez and Páez, 2000) or mid level (Hinestroza and Páez, 2001) of this beach. This spatial distribution shift might have been the result of a local dynamic of gain and loss of sand on the beach profile, where serious erosion is occurring.
During the 2008 nesting season, a total of 55 females were encountered but only four (2.2 %) were intercepted a second time. This low rate of recapture may have been due to several factors: i) there may be a high percentage of older females at this rookery, who lack the reproductive ability to renest. This would reflect a demographic imbalance in the population that has resulted from a low rate of juvenile recruitment (Hirth, 1971; Rueda, 1992) due to the anthropogenic pressures this site has experienced over the years (e.g., harvest of reproductive females and eggs). Mark-recapture efforts conducted during the 1998 season also only managed to document renesting in three females. ii) Females at the El Valle beach nest more than once, but do so in nearby beaches like the San Pichi beach, located south of the El Valle beach within the Utría Natural National Park. iii) Given the length of the beach and the extended time required to patrol it, some females may have been able to re nest undetected. However, this seems unlikely, since over 12 people working each night insured extensive coverage of the entire beach over the 2008 nesting season.
The average CCL reported by Hinestroza and Páez (2001) was 66 ± 2.47 cm, compared to 64.9 ± 2.4 cm (range 59.2 - 70.5; n = 52) in this study, and the average clutch size reported by Hinestroza and Páez (2001) was 102 ± 23.04 eggs, compared to 87 ± 19.1 eggs in this study. However, we feel more comparable data is needed from additional nesting seasons before it is possible to speculate that the anthropic pressures this rookery faces is producing a reduction in mean female size, and hence also mean clutch size. Low recapture rates between seasons also make it difficult to establish body growth rates for this population (Chaloupka and Musick, 1997).
The inter-nesting interval for olive ridley turtles is variable, but usually ranges between 14 to 28 days (Pritchard, 1969; Kalb and Owens, 1994; Plotkin, 1994), consistent with the 16 to 25 day intervals documented (n = 4) documented at the El Valle beach rookery. Although Carr (1967) reported that C. mydas lays fewer eggs in the first and last clutches each season, we observed a decrease in clutch size in the second clutch of all four re-nesting females, a trend also observed by Carr and Hirth (1962) and Pritchard (1971). However, we found that this reduction in the number of eggs per clutch was not significant.
We documented a significant relationship between clutch size and the CCL of nesting females. This pattern also occurs in green turtles (Carr and Hirth, 1962; Pritchard, 1969; Hirth, 1971) and loggerhead turtles (Ehrhart, 1995). Nevertheless, there was not a significant relationship between CCL and the total estimated reproductive output over the entire season, perhaps because of the small sample sizes this year.
There was a difference of 14 days in mean incubation periods between the 2008 and the 1999 seasons. We estimated hatching success at 81.1 % (± 12.1), representing an increase of 14.1 % compared to 1997 (Vélez, 1997), a decrease of 0.4 % compared to 1998 (Martínez and Páez, 2000) and an increase of 9.7 % compared to 1999 (Hinestroza and Páez, 2001) (Table 1.). The main mortality during incubation occurred during the embryonic development stage (34.1 %). While all years enjoyed high hatching success rates, but it will be necessary in future nesting seasons at the El Valle beach to compare success rates of in situ and transferred nests, in order to make better informed management decisions for this species.
CONCLUSIONS
Although the El Valle beach is considered one of the most important nesting sites in Pacific South America for L. olivacea, because of limited monitoring of nesting females, data are insufficient to establish population trends. Thus, the data obtained in this study is a valuable contribution to the knowledge of the reproductive ecology of the olive ridley regionally and globally, and hopefully will help refine on-going conservation actions. In 2008 we saw a substantial reduction in nest poaching by humans. According to data reporting from other seasons, when many females were harvested and poaching of nests approached 100 %, while in 2008 only five female turtles were consumed and egg poaching was only 2 % of all clutches. This demonstrates that continuous education efforts directed at members of the local community have produced greater environmental awareness and that local communities are able to develop respect for sea turtles and help efforts directed towards their conservation.
ACKNOWLEDGEMENTS
We thank the community council of El Cedro, the Fundacion Natura and the Utria Natural National Park for their contribution to the logistics of the project. Special thanks to Luis, Julio and Segundo Rivas, who were crucial in the data collection process and in protection of the beach and hatchery. We also thank local researchers (Asproplacu) for their collaboration with data collection and protection of the beach and hatchery. We thank Brian C. Bock provided comments and suggestions on the draft that improved the quality of the manuscript. Financial support for this study was obtained from ANECB, Medellin chapter. The metal flipper tags were provided by NOAA (National Marine Fisheries Service) under the Pacific Sea Turtle Flipper Tagging Program. The authors wish to acknowledge the use of the Maptool program for analysis and graphics in this paper. Maptool is a product of seaturtle.org (Information is available at www.seaturtle.org). In addition, we sincerely thank our handling editors, as well as the three anonymous reviewers of this paper.
REFERENCES
Álava JJ, Pritchard P, Wyneken J, Valverde H. First documented record of nesting by the olive ridley turtle (Lepidochelys olivacea) in Ecuador. Chelonian Conserv Biol. 2007;6(2):282-285. DOI: http://dx.doi.org/10.2744/1071-8443(2007)6[282:FDRONB]2.0.CO;2 [ Links ]
Amorocho DH, Rubio Diaz R. Observaciones sobre el estado actual de las tortugas marinas en el Pacífico Colombiano. In: Machecha R, Sánchez H, editors. Contribución al Conocimiento de las Tortugas Marinas de Colombia. Serie de publicaciones especiales de INDERENA, Bogotá; 1992. p. 155-179. [ Links ]
Amorocho D. Biología reproductiva de la tortuga golfina en Playa Larga, El Valle, Chocó. Informe Técnico. 1993. p. 91-92. [ Links ]
Barrientos K, Ramírez C. Estado actual de Lepidochelys olivacea en el Valle, Pacifico Chocoano, Colombia. In: Kelez S, van Oordt F, de Paz N, Forsberg K, editors. Libro de Resumenes. II Simposio de Tortugas Marinas en el Pacifico Sur Oriental. 2008. p. 7-21. [ Links ]
Barrientos-Muñoz KG, Ramírez-Gallego C, Rivas L. First Report on the Nesting of Black Sea Turtle (Chelonia mydas) on the North Pacific of Colombia. MTN. 2013;138:19-21. [ Links ]
Carr A. So Excellent a Fishe. New York: Natural History Press. 1967. p. 136-138. [ Links ]
Carr A, Hirth H. The ecology and migration of sea turtles, 5. Comparative features of isolated green turtle colonies. Am Mus Novit. 1962;(2091):1-42. [ Links ]
Ceballos-Fonseca C, Martínez L, Quiroga D. Distribución, amenazas y esfuerzos de conservación de las tortugas marinas en el Pacifico Colombiano. Informe final, Invemar, Santa Marta, Colombia. 2003. p.13-27. [ Links ]
Chacón D, Sánchez J, Calvo JJ, Ash J. Manual para el manejo y la conservación de las tortugas marinas en Costa Rica; con énfasis en la operación de proyectos en la playa y viveros. Primera edición. Asociación ANAI, Sistema Nacional de Àreas de Conservación (SINAC), Ministerio de Ambiente y Energia (MINAE). Gobierno de Costa Rica. San José, Costa Rica; 2007. p. 41-78. [ Links ]
Chaloupka M, Musick J. Age, Growth and population Dynamics. In: Lutz P, Musick J,Wyneken J, editors. Biology of Sea Turtles, Vol. II. CRC Press. 1997. p. 233-276. [ Links ]
Chaves G, Morera R, Avilés JR, Castro JC, Alvarado M. Trends of the nesting activity of the "Arribadas" of the olive ridley (Lepidochelys olivacea), Eschscholtz 1829, in the Ostional National Wildlife Refuge. 1971-2003. San José, Costa Rica; 2005. p. 23-39. [ Links ]
Cornelius S. Marine turtle nesting activity at Playa Naranjo, Costa Rica. Brenesia. 1976;8:1-27. [ Links ]
Eckert K, Bjorndal K, Abreu-Grobois F, Donnelly M. Research and Management Techniques for the Conservation of Sea Turtles. IUCN/SSC Marine Turtle Specialist Group Publication No. 4. Washington, D.C.; 1999. p. 232. [ Links ]
Eguchi T, Gerrodette T, Pitman RL, Seminoff JA, Dutton PH. At-sea density and abundance estimates of the olive ridley turtle Lepidochelys olivacea in the eastern tropical Pacific. Endang Species Res. 2007;3:191-203. [ Links ]
Ehrhart L. A review of sea turtle reproduction. In: Bjorndal K editors. Biology and Conservation of Sea Turtles. Smithsonian Institution Press. Washington, USA; 1995. p. 29-38. [ Links ]
Fretey J. Biogeography and conservation of marine turtles of the Atlantic coast of Africa. CMS Technical Series Publication UNEP/CMS Secretariat, Bonn. 2001. p. 429. [ Links ]
Hays-Brown C, Brown WM. Status of sea turtles in Southeastern Pacific: Emphasis on Peru. In: Bjorndal KA, editors. Biology and Conservation of Sea Turtles. Smithsonian Press, Washington D.C; 1982. p. 235-240. [ Links ]
Hinestroza L, Páez V. Anidación y manejo de la tortuga golfina (Lepidochelys olivacea) en la playa La Cuevita, Bahía Solano, Chocó, Colombia. Cuad Herpetol. 2001;14(2):131-144. [ Links ] [ Links ]
IUCN 2013. IUCN Red List of Threatened Species. Version 2013.2. Available at: www.iucnredlist.org. Downloaded on 01 March 2014. [ Links ]
Kalb H, Owens D. Differences between solitary and arribada nesting olive ridley females during the internesting period. In: Bjorndal KA, Bolten B, Johnson DA, Eliazar PJ, editors. Proceedings of the 14th Annual Symposium on Sea Turtle Biology and Conservation. NOAA Technical Memorandum NMFS-SEFSC-351; 1994. p. 68. [ Links ]
Kelez S, Velez-Zuazo X, Angulo F, Manrique C. Olive ridley Lepidochelys olivacea nesting in Peru: The southernmost records in the Eastern Pacific. MTN. 2009;126:5-9. [ Links ]
Manrique C, Kelez S, Velez-Zuazo X. Hatchlings in Peru: the first headstarting experience, In: Seminoff JA, editors. Proceedings of the Twenty-Second Annual Symposium on Sea Turtle Biology and Conservation. NOAA Technical Memorandum NMFS-SEFSC-503; 2003. p. 99. [ Links ]
Marcovaldi MA. Status and distribution of the olive ridley turtle, Lepidochelys olivacea, in the Western Atlantic Ocean. In: Eckert KL and Abreu Grobois FA, editors. Proceedings of the Regional Meeting "Marine Turtle Conservation in the Wider Caribbean Region: A Dialogue for Effective Regional Management" Santo Domingo, 16-18 November 1999. WIDECAST, IUCN-MTSG, WWF and UNEP-CEP; 2001. p. 52-56. [ Links ]
Márquez M.R, Peñaflores C, Vasconcelos J. Olive ridley turtles (Lepidochelys olivacea) show signs of recovery at Escobilla, Oaxaca. MTN. 1996;73:5-7. [ Links ]
Márquez MR. Sea Turtles of the World. FAO Species Catalogue. Rome: Food and Agricultural Organization of the United Nations; 1990. p. 11-81. [ Links ]
Martínez LM, Páez VP. Ecología de anidación de la Tortuga golfina (Lepidochelys olivacea) en la Playa de La Cuevita, Costa Pacífica Chocoana, Colombia, en 1998. Actual Biol. 2000;22(73):131-143. [ Links ]
Miller J. Determining Clutch Size and Hatching Success. In: Eckert K, Bjorndal KA, Abreu-Grobois FA and Donnelly M, editors. Research and Management Techniques for the Conservation of Sea Turtles. IUCN/SSC Marine Turtle Specialist Group Publication No. 4. Washington, D.C; 1999. p. 124-129. [ Links ]
Plotkin P. Migratory and reproductive behaviour of the olive ridley turtle, Lepidochelys olivacea. Eschscholtz, 1829, in the eastern Pacific Ocean (PhD Thesis). Texas: College Station, Texas A and M University; 1994. p. 29-40. [ Links ]
Prahl H, Cantera J, Contreras R. Manglares y hombres del Pacífico colombiano. Fondo FEN. Bogotá, Colombia; 1990. p. 30. [ Links ]
Pritchard PCH. Studies of the systematics and reproductive cycles of the genus Lepidochelys (PhD Thesis). Gainesville: University of Florida; 1969. p. 20-73. [ Links ]
Pritchard PCH. Galapagos sea turtles - preliminary findings. J Herp. 1971;5(1-2):1-9. [ Links ]
Pritchard PCH. Evolution, phylogeny, and current status. In: Lutz PL, Musick JA, editors. The Biology of Sea Turtles. Florida: Ediciones CRC Marine Science Series; 1997. p.1-28. [ Links ]
Pritchard PCH, Plotkin PT. Olive ridley sea turtle, Lepidochelys olivacea, In: Plotkin PT, editors. National Marine Fisheries Service and U. S. Fish and Wildlife Service Status Reviews for Sea Turtles Listed under the Endangered Species Act of 1973. Maryland: National Marine Fisheries Service, Silver Spring; 1995. p. 123-139. [ Links ]
R Development core team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria; 2013.
Ramírez C, Barrientos K. Nesting activity of the olive ridley (Lepidochelys olivacea) at El Valle, Chocoan Pacific coast, Colombia. In Jones T and Wallace B, compilers. Proceeding of the Thirty-first Annual Symposium on Sea Turtle Biology and Conservation. NOAA Technical Memorandum NOAA NMFS-SEFSC-631; 2012. p. 60-61. [ Links ]
Reichart HA. Synopsis of Biological Data on the Olive Ridley Sea Turtle Lepidochelys olivacea (Eschscholtz 1829) in the western Atlantic. NOAA Tech. Memo. NMFS-SEFSC-336. U.S. Dept. of Commerce; 1993. p. 78. [ Links ]
Reina R, Spotila J, Mayor P, Piedra R, Paladino F. Nesting ecology of the leatherback turtle, Dermochelys coriacea, at Parque Nacional Marino Las Baulas, Costa Rica: 1988-89 to 1999-2000. Copeia. 2002;3:653-664. [ Links ]
Rueda J. Anotaciones sobre un caso de mortalidad masiva de tortugas marinas en la costa pacífica de Colombia. In: Machecha R, Sánchez H, editors. Contribución al Conocimiento de las Tortugas Marinas de Colombia. Serie de publicaciones especiales de INDERENA, Bogotá; 1992. p. 22. [ Links ]
Spotila JR. Sea turtles: A complete guide to their biology, behavior, and conservation. Baltimore: The Johns Hopkins University Press; 2004. p. 240. [ Links ]
Valverde RC, Gates CA. Population surveys on mass nesting beaches. In: Eckert K, Bjorndal KA, Abreu-Grobois FA and Donnelly M, editors. Research and Management Techniques for the Conservation of Sea Turtles. IUCN/SSC Marine Turtle Specialist Group Publication No. 4. Washington, D.C; 1999. p. 64-69. [ Links ]
Vélez A. Informe técnico sobre la temporada de anidación de 1997. Presentado a Fundación Natura, Bogotá; 1997. p. 5. [ Links ]
Wester J, Kelez S, Velez-Zuazo X. Expanding nesting ranges: the southernmost records of Chelonia mydas and Lepidochelys olivacea nesting in the Eastern Pacific. In Jones T and WallaceB, compilers. Proceeding of the Thirty-first Annual Symposium on Sea Turtle Biology and Conservation. NOAA Technical Memorandum NOAA NMFS-SEFSC-631; 2011. p. 72. [ Links ]