Introduction
Lung cancer (LC) is the main cause of cancer-related death in men and women worldwide with high lethality 1. In Colombia, for the year 2020, the incidence was 2.8, and mortality was 3.0 per 100,000 inhabitants, respectively 2. The LC is divided into Small Cell Carcinoma and Non-Small Cell Carcinoma (NSCLC), the most frequent subtype (85%) 3.
Frequently, NCSLC is diagnosed at an advanced-stage disease 4. When deciding on treatment, it is important to consider factors such as age, stage at diagnosis, performance status, histological type and subtype, and the presence of recurrent genomic alterations 5,6.
Currently, the treatment of advanced or metastatic NSCLC has evolved over the years as a result of continuous improvement in clinical outcomes 7,8. For patients with driver mutations such as EGFR and ALK, first-line therapy should include targeted therapy with an effective drug against the detected alteration. This strategy has shown better outcomes compared to conventional chemotherapy 9-13. For patients without driver mutations, treatment is based on the combination of chemotherapy with immunotherapy or immunotherapy-only for patients with very high tumor PDL1 expression (≥50%) 14,15. In case of contraindication to immunotherapy, chemotherapy remains the mainstay of treatment 5.
Real-life evidence may corroborate the results obtained in clinical trials thus providing a solid the basis for ongoing use of treatment strategies recommended in current guidelines for advanced NSCLC patients. In Colombia, the incorporation of the best evidence is not always possible, as there are both regulatory and access barriers. So far, it is unknown if the incorporation of new technologies in other countries can be generalized to the Colombian population.
The evidence of the benefit of treatment strategies in the “real-life” scenario in Colombia has not been reported, and this provides the main justification for this endeavor. This study will describe critical time-to-event data of NSCLC patients treated at the Clínica de Oncología Astorga (COAstorga), an outpatient tertiary center that provides specialized cancer care in Medellín, Colombia.
Methods
This is an observational study with survival analysis. Patients diagnosed with advanced NSCLC (Stages IIIB and IIIC tumor [not amenable for curative therapy] or stage IV) from January 2019 to January 2022 (inclusive), who must have received systemic therapy at the COAstorga were included. Additionally, to be included, each patient had to approve the collection of his or her personal data and medical information through a habeas data format.
The sample size was for convenience. All patients who meet the eligibility criteria were analyzed.
Study variables include socio-demographic information (sex, age at diagnosis, type of health insurance affiliation, and site of residence), smoking history, date and method of diagnosis, tumor pathology (histology, grade of differentiation, and biomarkers), clinical stage at diagnosis, Eastern Cooperative Oncology Group (ECOG) Performance Status at diagnosis, data of treatment (date of treatment initiation, and first-line treatment).
We also evaluated prognostic variables (vital status, and dates of progression/recurrence/relapse/death). Disease relapse/progression was defined according to clinical criteria (based on physical examination and imaging). The date of death was obtained from medical records. If the data was not available, the data was obtained from the ADRES (Administradora de los Recursos del Sistema General de Seguridad Social en Salud) database, a government source. Since the data was typically unavailable, we did not mention the cause of death.
The Research Unit staff collected the study data from the patients’ medical records, both retrospectively and prospectively. Subsequently, the staff validated the data under the supervision of the Principal Investigator and the Research Director.
Study outcomes were: Overall survival (OS) was defined as the length of time (months) from date of diagnosis until last follow-up or death from any cause (OS1), or from start of cancer treatment until the date of the last follow-up contact or the date of death from any cause (OS2). And, progression-free survival (PFS), is the length of time (months) from the start date of cancer treatment until the date of disease progression or the date of death from any cause.
For statistical analysis, quantitative variables are described using median and interquartile range (IQR). Categorical variables are expressed as frequencies and percentages. Kaplan Meier method was used to estimate OS1, OS2, and PFS. To compare the survival distributions log-rank test was performed. Subgroup analysis was carried out according to histology, ECOG status at diagnosis, and type of treatment received. Statistical analysis was performed using SPSS version 22.
Results
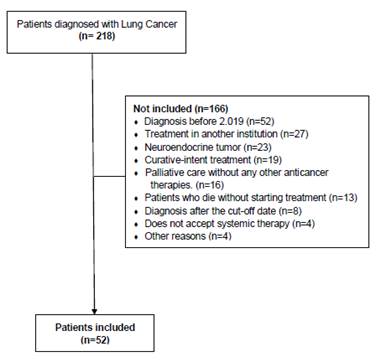
Fifty-two patients were included. Patient flow chart (Figure 1) shows the selection of patients. Median age was 70.1 years (IQR: 64.6-77.2), and 57.7% were men. Concerning affiliation to the Colombian Healthcare system, 88.5% of patients belonged to the Contributive Regime (Table 1).
In all cases, tissue biopsy was the diagnostic method for LC. Histology was divided into two groups: squamous cell carcinoma (SCC) and non-squamous cell carcinoma (NSqC), including adenocarcinoma. Adenocarcinoma was the histology in 57.7%. Patients with adenosquamous histology were classified in the NSqC group. For NSqC tumors a positive PD-L1 expression was observed in 11/27 (40.7%) patients, ALK-positive in 3/28 (10.7%), and EGFR mutation in 3/29 (10.3%). In SCC histology, positive PD-L1 expression and ALK-rearrangements were found in 4 out of 7 (57.1%), and in 1 out of 1 ordered (100.0%), respectively. No EGFR mutation was observed (Table 1). The median follow-up (from the time of diagnosis to date of death or to date of the last patient follow-up) was 10.0 months (IQR: 4.9-18.5). At diagnosis, stage IV was established in 88.5%.
Treatments were divided into three groups: Chemotherapy, immunotherapy, and tyrosine kinase inhibitors (TKI). Chemotherapy (chemotherapy with or without anti-angiogenic therapy) was the first line treatment in 25 (48.1%) patients, 21 (40.4%) patients underwent immunotherapy with or without chemotherapy, 5 (9.6%) patients received TKI, and 1 patient was enrolled in a clinical trial (Table 2).
Table 1 Sociodemographic and clinical characteristics of patients with advanced Non-Small Cell Lung Cancer.
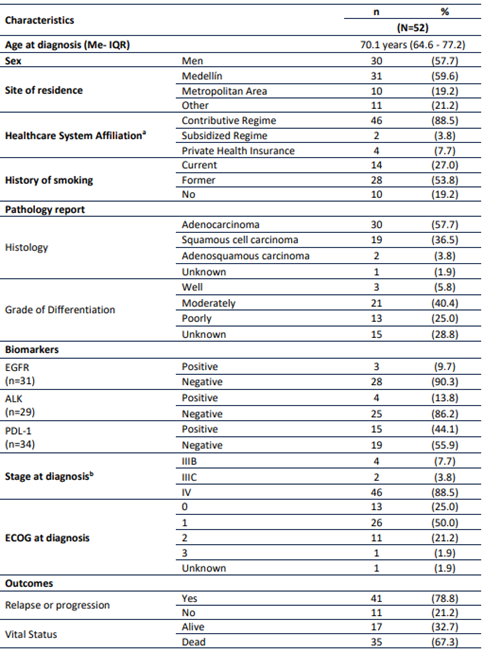
Me: Median; IQR: Interquartile Range. EGFR: Epidermal growth factor receptor. ALK: Anaplastic lymphoma kinase. PD-L1: Programmed death-ligand 1. ECOG: Eastern Cooperative Oncology Group (ECOG) Performance Status. aAffiliation type to the healthcare system: Contributive Regime is co-financed by employees and employers, Subsidized Regime covers the lowest income population and it is financed with government. Private Health Insurance: Individuals who pay for complementary health insurance.
Table 2 First-line treatment of patients with advanced Non-Small Cell Lung Cancer.
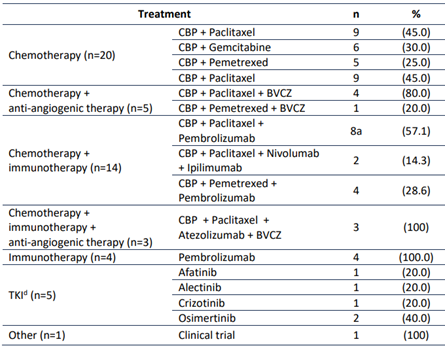
CBP: Carboplatin. BVCZ: Bevacizumab. TKI: Tyrosine kinase inhibitors. a One patient underwent radiotherapy followed by chemotherapy with immunotherapy.
Afatinib and osimertinib were the first-line treatment in patients with EGFR mutations. Alectinib and crizotinib were used in ALK-positive patients. Of the 4 patients with ALK-positive, 2 patients did not receive target therapy because molecular information was not available for therapeutic decision. In these cases, chemotherapy and immunotherapy with chemotherapy were administered.
Twenty-five patients received chemotherapy (with or without antiangiogenic agent) without immunotherapy as first-line. In 13 (52.0%), immunotherapy was not prescribed due to performance-status 2 or more. In 3 (12.0%) each, the treating physician did not recommend immunotherapy for PD-L1 negative disease or chemotherapy was initiated and immunotherapy was withheld while waiting for biomarker results. In 2 (8.0%), immunotherapy was prescribed but access was not granted by the insurer. In 1 (4.0%) each, immunotherapy was administered immediately after chemo-radiotherapy or the patient had an autoimmune condition deemed a contraindication to immunotherapy. And in 2 (8.0%), the reasons for not prescribing immunotherapy are unknown.
At the time of analysis, progression was observed in 41 (78.8%) patients (Table 1). Median PFS was 6.0 months (95%CI: 4.8-7.1) (Figure 2A). At the time of analysis, 32.7% of the patients were alive. Median OS1 was 11.0 months (95%CI: 6.7-15.3) (Figure 2B).
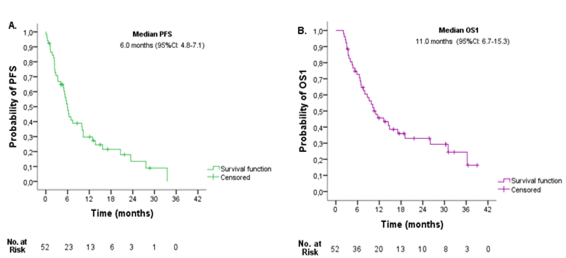
Figure 2 Entire cohort survival outcomes. A. Progression-free survival (PFS), and B. Overall survival from diagnosis (OS1).
According to histology, median PFS was 6.0 months (95%CI: 4.4-7.5) for patients with SCC, and 6.4 months (95%CI 0.9-11.9) for patients with NSqC (Figure 3A). Median OS1 were 7.0 months (95%CI: 2.0-11.9) and 14.5 months (95%CI: 4.6-24.4) for SCC and NSqC, respectively (Figure 3B). No significant difference in PFS (P=0.245) and OS were observed in histology groups (P=0.103).
Patients were divided into two groups according to ECOG PS scores at diagnosis (ECOG 0 or 1 and ECOG 2 or 3). Median PFS were 6.9 months (95%CI: 2.3-11.6) and 2.5 months (95%CI: 1.0-4.0) for patients with ECOG 0-1 and ECOG 2-3, respectively. Median OS1 was 14.5 months (95%IC: 7.3-21.7) for ECOG 0-1, and 4.7 (95%IC: 2.7-6.7) for ECOG 2-3. Significant difference in PFS (P=0.002)) and OS1 (P=0.002) were observed between groups.
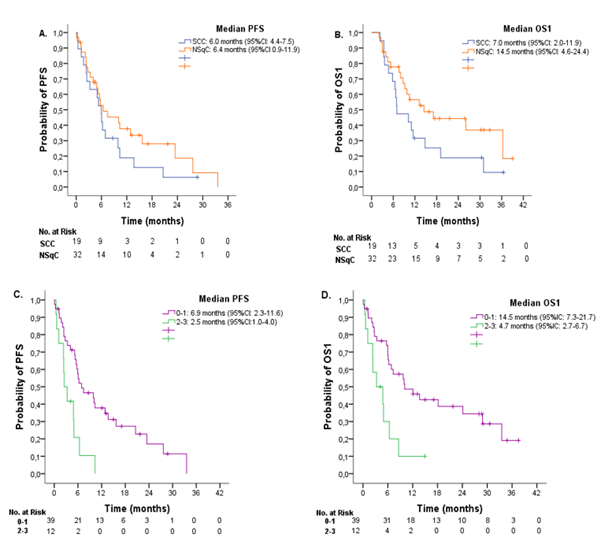
Figure 3 A. Progression-free survival (PFS) and B. Overall survival from diagnosis (OS1) according to histology in patients with advanced Non-Small Cell Lung Cancer. SCC= Squamous cell carcinoma. NSqC= Non-squamous cell carcinoma. C. PFS and D. OS1 according to Eastern Cooperative Oncology Group (ECOG) Performance Status.
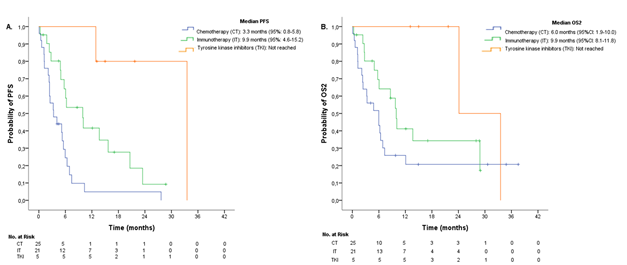
When stratifying according to treatment groups, median PFS for chemotherapy, immunotherapy, and TKI were 3.3 months (95%CI: 0.8-5.8), 9.9 months (95%CI: 4.6-15.2), and not reached at the time of analysis, respectively (Figure 4A). A significant difference in PFS was observed (P <0.001). Median OS2 was 6.0 months (95%CI: 1.9-10.0) for chemotherapy, 9.9 months (95%CI: 8.1-11.8) for immunotherapy, and was not reached for TKI (Figure 4B). No significant difference in OS2 was found between groups (P=0.080).
Discussion
In this report, we aim to describe the demographic and survival outcomes of a cohort of 52 patients with advanced NSCLC treated with systemic therapy at an outpatient oncology facility in Medellín, Colombia.
Males accounted for the majority of the patients with 57.7%. The median age was 70 years old. Tobacco was a significant risk factor accounting for 80.8%. Adenocarcinoma was the main histology, in line with current worldwide trends. Driver mutations in EGFR and ALK were detected in 13.5%. Unfortunately, a significant proportion of patients had poor performance status with ECOG ≥ 2 in 23.1% at the outset. Only 40.1% underwent first-line immunotherapy with anti PD1 or anti PD-L1 agents (with or without chemotherapy), and 2 out of 7 patients with actionable mutations did not have access to targeted therapy (28.6%).
Median PFS and OS in the whole cohort were 6.0 months and 11.0 months, respectively. These results in patients with performance status 0 and 1 are in line with those reported in the literature for metastatic, relapsed and recurrent NSLCC with PFS in the order of 9-17 months and OS in the range of 13-38 months depending on the treatment strategy and whether the patient is a candidate for targeted therapy 16-24. In the 12 patients with performance status 2-3 included in this analysis, median OS was 4.7 months. This result contrasts with median OS of 9.3 months and 6.3-6.9 months in platinum-based combination chemotherapy in randomized trials 25.
Our survival outcomes are affected by several factors, including older age at presentation, a significant proportion of poor-performance patients undergoing systemic therapy, and lack of access to targeted therapy for some potential candidates for this type of treatment. The use of immune checkpoint inhibitors in first-line was somewhat less than expected as these agents are recommended for almost all metastatic, relapsed, and recurrent NSCLC not amenable to anti-EGFR or anti-ALK therapy 7.
A strength of the study is the accurate description of the main treatment modalities in use, a feature not available in most registries in Colombia. Another strength of the study is the inclusion of patients with performance status 2 and beyond undergoing systemic therapy, a segment of the population frequently neglected in clinical trials that constitutes a significant minority of patients deemed candidates for systemic therapy. As expected, patients with performance status 2 and beyond have shorter PFS and OS.
There are limitations of this study: First, this study is retrospective in nature, it is possible that the totality of the data was not available at the time of collection. Due to its small sample size and given that patients were treated at a single institution these results cannot be extrapolated. However, they provide useful information about this condition in the population treated at the institution.
In conclusion, median progression-free survival and overall-survival in 52 advanced NSCLC treated in a real-world setting in Colombia, a Low-Middle-Income-Country, were 6 and 11 months, respectively. This data can better inform patients and providers alike in regards to expectations of survival in advanced NSCLC treated in Colombia. Advanced disease with poor performance status and lack of access to state-of-art therapy are challenges that need to be overcome.