Introduction
The study design guides the methodology to obtain the answer to the research question. However, as we learned in the previous article on “how to formulate a research question,” it is the question itself that determines the appropriate architecture, tactics, and strategy to be used1.
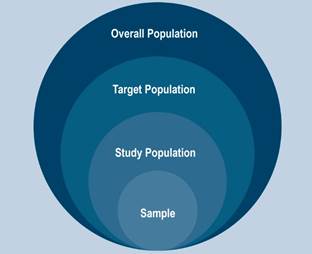
Research study designs can be broadly categorized into two groups: experimental and non-experimental, based on the level of manipulation performed by the researcher on the study population or sample. The sample, as depicted in Figure 1, consists of individuals selected (based on inclusion and exclusion criteria) from the accessible population, derived from the target population or characterized by the most important feature of our study (e.g., patients with gastric cancer, inflammatory bowel disease, or gastroesophageal reflux, among others)2,3.
Experimental studies
Experimental studies, also known as randomized studies, are characterized by researcher manipulation. This manipulation or intervention (such as medication, a device, or surgical intervention) is referred to as the independent variable, while the dependent variable is the final outcome intended to be demonstrated by the intervention (e.g., pain improvement, histological resolution, mortality, survival, among others)1,2. The aim of these experimental studies is to determine the cause-effect relationship between the intervention (independent variable) and the outcome (dependent variable) by comparing it to a randomized control group, thereby minimizing the risk of confounding factors. Within experimental studies, there is a subclass known as quasi-experimental studies, where the key difference is that there is intervention or manipulation but no randomization. These studies typically involve a single designated group or comparisons with previous studies or historical cases1,2.
The advantages of these studies are their simplicity and universal acceptance, their applicability across various fields, the ease of interpreting and analyzing their results, and their usefulness in all phases of studies and in meta-analyses. However, the disadvantages include their susceptibility to confounding factors, significant variability among patients if not properly selected, high cost, the general need for large sample sizes, and the challenge of reproducing results in real-world settings if the study is too controlled1-3.
Observational studies
The key characteristic of observational studies is that the researcher does not intervene in the exposure or independent variable. As a result, the relationship with the dependent variable is not controlled and is typically biased by other variables. These studies can be descriptive when there is no comparison group, or analytical when there is a comparison group, allowing for statistical inferences and relationships between exposures or risk factors and the dependent variable or expected outcome2,3.
Non-Analytical Observational Studies
Classic examples of this type are case reports and case series. These studies are very valuable, as they describe the disease history, clinical presentation, diagnostic approach, and treatment response. They generally involve rare diseases or presentations and sometimes report adverse drug events. These studies help to formulate or create hypotheses related to each case2,3.
The statistical analysis of these studies is limited, typically determining only symptomatic prevalences. Their advantages include identifying unknown clinical presentations, exploring new treatment possibilities, and generating new hypotheses about disease mechanisms or risk factors. However, their disadvantages are the scarcity of statistical data due to the absence of a control group, lack of efficacy or safety data, inability to determine associations, and high risk of publication bias1-3.
Analytical Observational Studies
These studies include control groups for comparison and encompass cross-sectional studies, case-control studies, and prospective and retrospective cohort studies.
Cross-Sectional Studies
Cross-sectional studies investigate the characteristics of a sample population at a specific point in time. Patients are randomized based on the development of the disease to determine whether exposure leads to the development of the disease or condition2,3.
The statistical analysis of these studies is based on odds and prevalence. Their advantage lies in their ability to determine disease prevalence and the measures of associations between exposure and disease1. However, in cases of rare or infrequent diseases, the disadvantages include potential overestimation in cases of long-duration diseases or underestimation in short-duration diseases, and the inability to establish causality due to the single-time-point nature of the study2-4.
Case-Control Studies
In case-control studies, patients or individuals in the case group are identified by the presence of the disease or specific characteristic (dependent variable). Controls are individuals from the same target population who do not have the disease or characteristic, and the researcher retrospectively examines the risk factors possibly related to the development of the disease. Statistical analysis is based on the odds ratio regarding exposure2,3.
The advantages of case-control studies include their cost-effectiveness in examining multiple risk factors simultaneously relying on a small sample and their suitability for studying rare diseases. However, disadvantages include the potential difficulty in accurately determining cases and controls based on diagnostic criteria (equal risk in the same population)2-4.
Cohort Studies
In cohort studies, individuals are categorized based on the presence of a risk factor or exposure and are followed over time to determine the development of the disease or characteristic (dependent variable). Participants are divided into two groups-exposed and non-exposed-and are followed in parallel, either prospectively or retrospectively, depending on the study design2-4.
The statistical analysis in these studies is based on disease risk and frequency. The primary advantage of cohort studies is the ability to directly observe the temporal sequence between exposure and disease development. However, the disadvantages include high cost, long duration, and a significant likelihood of loss to follow-up2-4.
Conclusions
To answer a research question, various study designs and research methods are outlined, highlighting their main characteristics and the most practical classifications for determining the best study design. Key aspects of the study population, advantages and disadvantages, and the internal and external validity of the studies are discussed to understand, based on the research question, the potential for extrapolating the results.











 text in
text in