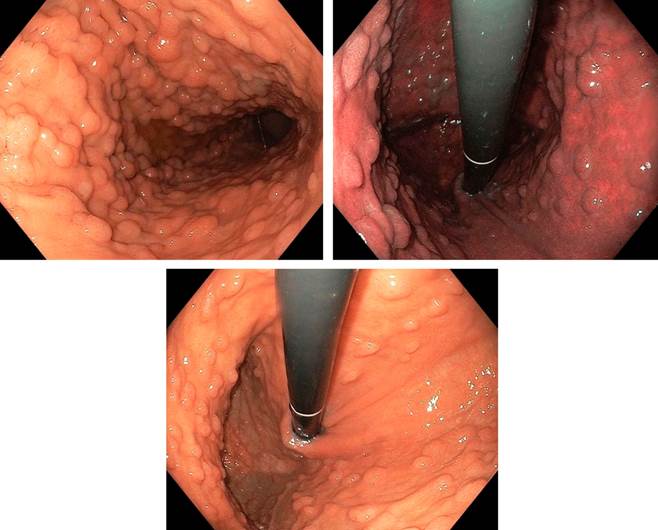
Case presentation
A 28-year-old female patient with a history of familial adenomatous polyposis (FAP) underwent a total proctocolectomy and creation of an ileal pouch six years ago. Biopsy confirmed the presence of adenocarcinoma, necessitating ongoing monitoring with reservoiroscopies. She also has a history of papillary thyroid carcinoma, which was surgically treated four years ago. In April 2023, an upper gastrointestinal endoscopy (UGIE) was performed.
The UGIE revealed no endoscopic alterations in the duodenum, the region corresponding to the papilla, the antropyloric region, and the angular zone. However, in the mid and upper zones of the gastric body and fundus, multiple polyps (more than 100), each smaller than 5 mm were observed. These polyps were scattered, some forming clusters, with mucosa similar to the surrounding area. Multiple biopsies were taken.
The biopsies indicated the presence of a fundic gland polyp, moderate chronic gastritis, follicular, with mild activity; these findings are consistent with Helicobacter pylori. There was no evidence of atrophy, metaplasia, or dysplasia. The result was negative for malignant cells (Figure 1A-C).
Discussion and literature review
FAP is an autosomal dominant hereditary syndrome caused by a mutation in the APC gene located on chromosome 5q21. While the primary manifestation of this disease is the presence of numerous colon adenomas, the upper gastrointestinal tract can also be affected. Patients with FAP have a significant risk of extracolonic malignancies, primarily in the duodenum, but also an increased risk of stomach cancer. Up to 20% to 30% of FAP cases are de novo, arising from a new pathogenic variant in APC, and therefore, may lack a family history of FAP1.
Endoscopic surveillance of the colon and prophylactic colectomy have significantly decreased colorectal cancer mortality and improved the survival of patients with FAP, resulting in a greater need for surveillance of extracolonic malignancies. Endoscopic surveillance in FAP patients is a safe and effective method for diagnosing precancerous lesions, allowing for the treatment of most benign lesions and early-stage cancers. However, surveillance recommendations for the digestive tract are not fully defined.
The initial recommendations for gastric polyp surveillance were based on the Spigelman classification, which in 1989 described a staging system for duodenal polyps to stratify the risk of duodenal cancer in FAP2.
Gastric lesions in FAP
Most patients with FAP develop gastric polyposis and have a higher risk of gastric cancer compared to the general population. According to the National Comprehensive Cancer Network (NCCN) Guidelines Report “Genetic/Familial High-Risk Assessment: Colorectal, Version 1.2022”, the estimated lifetime risk of gastric cancer in patients with a hereditary disease with APC mutation is 0.1% to 7.1% compared to 0.8% in the general population, with the average onset between 52 and 57 years. Cannon and colleagues, in their publication based on data from the U.S. polyposis registry, identify gastric cancer as the leading cause of death in patients with FAP and attenuated FAP (AFAP) after duodenal and ampullary cancers3,4.
Screening procedures with the removal of suspicious lesions can prevent the vast majority of carcinomas in these patients. Gastric lesions are common in adult patients with FAP; therefore, recognizing the different types of polyps, their early detection, and performing biopsies or removing them are very important in preventing gastric cancer in these patients.
Types of Gastric Polyps in Patients with FAP
These types include fundic gland polyps, gastric foveolar-type adenomas, gastric intestinal-type adenomas, pyloric gland adenomas, hyperplastic polyps, and gastric adenocarcinomas.
Fundic Gland Polyps (FGPs)
FGPs are the most common type of polyps, observed in 40% to 88% of patients with FAP5,6. Typically small (< 5 mm), sessile, multiple, and asymptomatic, FGPs are confined to the stomach. FGPs associated with FAP differ from sporadic FGPs, showing a more balanced sex distribution. They often exhibit low-grade dysplasia, although they are not considered precursor lesions for most gastric adenocarcinomas in FAP. There is no established causal link between H. pylori and FAP-associated FGPs5.
Adenomas can appear anywhere in the stomach but are more frequently found in the antrum. They are less common than FGPs in patients with FAP. Antral adenomas are typically flat, sessile, and subtle with a red villiform appearance, whereas those in the gastric body and fundus are more polypoid with a pale yellow surface, making them difficult to differentiate from FGPs.
In patients with FAP, endoscopists should maintain a high degree of suspicion for detecting gastric adenomas and take multiple biopsies. Virtual endoscopy techniques can be useful for detecting these lesions, especially flat ones and subtle mucosal changes. The use of Fujinon Intelligent Chromo Endoscopy (FICE) technology can identify dysplasia and differentiate between adenomatous and non-adenomatous polyps, significantly increasing the detection of adenomas in FAP patients. This can be extrapolated to virtual endoscopy techniques like NBI (Olympus) or iScan (Pentax)6.
Adenomas located in the antrum of the stomach should be removed via endoscopic submucosal dissection (ESD) or endoscopic mucosal resection (EMR) when there is a high degree of suspicion during esophagogastroduodenoscopy (EGD)3.
Pyloric Gland Adenomas (PGA)
Pyloric gland adenomas are rare epithelial polyps more frequently found in patients with autoimmune atrophic gastritis and FAP. They are most commonly located in the gastric body and predominantly occur in women. PGAs are clinically significant due to their malignant potential, unlike hyperplasia of metaplastic glands. In some cases, high-grade dysplasia is observed, and invasive carcinoma is associated with 12% to 42% of these lesions7.
As with other types of adenomas, complete excision of PGAs with biopsy of the surrounding flat mucosa is appropriate in patients with FAP, as PGAs often arise in the context of chronic lesions.
Hyperplastic Gastric Polyps
These polyps are most prevalent in regions with a high incidence of H. pylori infection. In contrast, in Western countries, where H. pylori prevalence is lower and proton pump inhibitor (PPI) use is common, fundic gland polyps are more frequent. The British Society of Gastroenterology (BSG) guidelines suggest that hyperplastic polyps larger than 1 cm, pedunculated polyps, and those causing symptoms (obstruction, bleeding) should be resected. If H. pylori is present, it should be eradicated before re-evaluation. The guidelines also recommend that if adenomas or hyperplastic polyps are present, the fundic mucosa should be endoscopically evaluated for gastric atrophy, intestinal metaplasia, H. pylori, and synchronous neoplasia8.
Inflammatory Fibroid Polyps
These are rare lesions, representing less than 0.1% of all gastric polyps. After resection, inflammatory fibroid polyps generally do not recur, and surveillance is not recommended9.
Gastric Adenocarcinoma
Gastric adenocarcinoma develops from an adenoma and can occur anywhere in the stomach, potentially being multicentric and metachronous. Studies have shown that gastric cancer tends to appear around two decades after colectomy in FAP patients10.
Endoscopically, three features are most frequently observed in individuals with gastric cancer and FAP: solitary gastric polyps ≥ 2 cm, a carpet of proximal gastric polyps, and polypoid mounds within a proximal carpet. Another high-risk gastric lesion in FAP is the presence of white gastric plaques, which may reflect underlying adenomatous mucosa and likely indicate a higher risk of gastric cancer11-13.
In its 2020 guidelines on the role of endoscopy in FAP, the American Society for Gastrointestinal Endoscopy (ASGE) recommends surveillance every three to six months with random biopsies of polyps and endoscopic resection of polyps > 10 mm, particularly in cases of diffuse gastric polyposis and large clusters of polyps. The ASGE also recommends the endoscopic removal of all polyps located in the antrum due to the high likelihood of adenomas5,9.
The 2015 ASGE guidelines on the role of endoscopy in managing premalignant and malignant conditions of the stomach state that sampling gastric polyps with forceps may not reveal dysplastic components. These guidelines recommend complete polypectomy with a snare based on size: fundic polyps > 10 mm, hyperplastic polyps > 5 mm, and all adenomatous polyps. According to the ASGE, surgery should be reserved for patients with FGPs and adenomas harboring advanced histological features that fail endoscopic management. Additionally, they note that mucosal biopsy sampling may not be adequate for assessing malignancy within layers of carpeted polyposis or polypoid mounds and suggest that endoscopic ultrasound (EUS) may help evaluate underlying malignancy5.
It is crucial to address the extracolonic manifestations of FAP as part of a comprehensive healthcare plan due to the associated risks of duodenal/ampullary, thyroid, and gastric cancer14.
Surveillance
The current surveillance guidelines for the upper digestive tract from the American College of Gastroenterology (ACG) and the BSG/Association of Coloproctology of Great Britain and Ireland (ACPGBI)/UK Cancer Genetics Group, published in 2015 and 2020 respectively, strongly recommend initiating upper digestive tract surveillance between the ages of 25 and 30, with intervals every 0.5 to 4 years based on the Spigelman classification of duodenal polyposis. The NCCN guidelines recommend starting upper gastrointestinal endoscopy slightly earlier, between the ages of 20 and 25, due to concerns about the morbidity and mortality associated with duodenal/periampullary cancers in FAP. Gastrectomy may be considered if high-risk lesions cannot be removed endoscopically; ideally, these procedures should be performed in a specialized center experienced in managing FAP and should take patient-specific factors into account.
Additionally, if a patient with FAP needs to undergo a colectomy at an age younger than that recommended for starting upper gastrointestinal tract surveillance, an initial upper gastrointestinal endoscopy should be performed to rule out gastric neoplasia before the colectomy8,14,15.
Since the introduction of genetic testing and prophylactic colectomy in FAP, the incidence and mortality from colorectal cancer have decreased, and the detection of extracolonic cancer is recommended2,3.
Current EGD surveillance recommendations do not consider gastric polyposis but are determined by the duodenal stage of polyposis. It is suggested that EGD surveillance intervals should reflect the emerging risk of gastric cancer.
Mankaney and colleagues identified several endoscopic factors in FAP patients who developed gastric adenocarcinoma. As Bianchi suggests, gastric surveillance should include multiple random biopsies of polyps, targeted biopsy of unusually appearing proximal polyps, and snare resection of individual polyps > 10 mm or lesions in the antrum. The severity of gastric polyposis should be based on the size, number, and pathology of gastric polyps. A surveillance interval of three years is recommended for patients with few to numerous small FGPs or FGPs with foveolar low-grade dysplasia (LGD).
In the presence of a proximal gastric polyposis carpet, an EGD should be performed at an interval of one year, and more frequently depending on the size of solitary polyps, the presence of polypoid mounds, and the histology of the polyps. For patients with proximal gastric polyposis mounds, initial EUS with fine-needle aspiration (FNA) of suspicious lesions and endoscopic reduction of the polypoid mounds should be conducted, with follow-up every three to six months based on the pathology. A baseline magnetic resonance imaging or CT scan is recommended to check for metastatic disease when polypoid masses are found, due to the frequent occurrence of metastatic disease. If any pathology sample indicates high-grade dysplasia (HGD), gastrectomy should be recommended (Table 1).
Table 1 Follow-up of Gastric Adenomas in FAP
| Number of Polyps, Size, and Presence of Mound Polyps | Histology | Follow-Up Strategy |
|---|---|---|
| Multiple, < 10 mm | FGP with or without foveolar LGD | EGD according to Spigelman if duodenal polyp or every 3 years |
| Multiple or carpet, < 10 mm | PGA or TA | Every year |
| Multiple or carpet, > 10 mm | FGP with or without foveolar LGD, PGA, or TA | Every 6 to 12 months |
| Multiple of any size without mound formation | FGP with HGD, PGA with HGD, or TA with HGD | Every 3 to 6 months or offer gastrectomy |
| Proximal polyp mounds | FGP with or without foveolar LGD, PGA, or TA | Every 3 to 6 months, EUS. Consider CT scan or MRI of the abdomen |
| Proximal polyp mounds | FGP with HGD, PGA with HGD, or TA with HGD | Prophylactic gastrectomy |
| Any size or number | Intramucosal or invasive adenocarcinoma | Gastrectomy |
PGA: pyloric gland adenomas; TA: tubular adenoma; HGD: high-grade dysplasia; LGD: low-grade dysplasia; EUS: endoscopic ultrasound; MRI: magnetic resonance imaging; CT: computed tomography. Taken from Mankaney GN and colleagues; Endosc Int Open. 2022;10(08):E1080-E79.
Patients with numerous proximal polyposis or carpet without polypoid mounds and with FGP-HGD, PGA-HGD, or TA-HGD should be reviewed every three months or offered prophylactic gastrectomy. Any patient with intramucosal carcinoma or invasive cancer should undergo gastrectomy9.
The last-mentioned work is an effort to standardize the follow-up of gastric lesions in patients with FAP. However, to date, there are no recommendations from international societies on this matter, so each case must still be individually analyzed, and studies with higher-quality evidence are needed to establish accurate follow-up guidelines.











 text in
text in