Introduction
Celiac disease (CD) is a systemic immune-mediated disorder, characterized by a specific serological and histological profile associated with gluten intake, and a broad range of gastrointestinal and extra-gastrointestinal clinical manifestations1. It affects approximately 1% of the global population, although most cases remain undiagnosed due to nonspecific symptoms and a general lack of awareness about the disease. The incidence and prevalence of CD are on the rise, likely due to industrialization, increased consumption of gluten-containing foods, heightened clinician awareness for diagnosing the disease, and advancements in diagnostic methods2. CD affects individuals of various ages, races, and ethnic groups. The epidemiological behavior of this disorder in Colombia is largely unknown, with very few reported cases and even fewer published studies on the topic3-5.
Case presentation
A 55-year-old female patient with no relevant medical history presented with a three-week clinical course of early satiety, weight loss, intolerance to legumes, abdominal distension, and chronic watery diarrhea, along with paresthesias initially in the upper limbs and subsequently affecting the lower limbs. During the systems review, the patient reported experiencing similar symptoms for the past two years, accompanied by a decrease in functional class due to dyspnea with moderate to minor exertion and an unintentional weight loss of approximately 20 kg over the same period. Due to the exacerbation of symptoms, she sought emergency care. On physical examination, her vital signs were normal, her weight was 34 kg, and notable findings included pale oral mucosa, a smooth and geographic tongue, and epigastric pain upon deep palpation, without signs of peritoneal irritation.
Laboratory tests revealed macrocytic hyperchromic anemia, leukopenia, thrombocytopenia, normal renal function, hypokalemia, hypocalcemia, hypomagnesemia, prolonged international normalized ratio (INR), and deficiencies in vitamins B12 and D (Table 1). Intravenous electrolyte replacement was administered, and additional studies were conducted. Endoscopic studies of the upper and lower digestive tracts, tumor markers, and thoracic and abdominal CT scans were unremarkable.
Table 1 Admission Laboratory Results
| Laboratory Test | Result | Reference Values |
|---|---|---|
| Hemogram | ||
| Leukocytes | 2.1 x 103/μL | 4.5-10 x 103/μL |
| Red blood cell count | 3.8 x 106/μL | 4.2-5.4 x 106/μL |
| Hemoglobin | 6.6 g/dL | 12.5-16 g/dL |
| Hematocrit | 18.90% | 37%-47% |
| Mean corpuscular volume | 129.5 fL | 79-101 fL |
| Mean corpuscular hemoglobin | 35.2 pg | 29-35 pg |
| RDW-CV | 12.00% | 11%-16% |
| Platelets | 58 x 103/μL | 150-450 x 103/μL |
| Electrolytes | ||
| Potassium | 2.7 mmol/L | 3.5-5.1 mmol/L |
| Sodium | 141 mmol/L | 136-146 mmol/L |
| Chloride | 106 mmol/dL | 101-109 mmol/L |
| Magnesium | 1.15 mg/dL | 1.9-2.5 mg/dL |
| Serum calcium | 5.4 mg/dL | 8.9-10.3 mg/dL |
| Renal function | ||
| Creatinine | 0.56 mg/dL | 0.55-1.02 mg/dL |
| Blood urea nitrogen | 8 mg/dL | 7-25 mg/dL |
| Liver function | ||
| AST | 38 U/L | 0-35 U/L |
| ALT | 31 U/L | 0-35 U/L |
| INR | 1.46 | 0.8 a 1.2 |
| Albumin | 3 g/dL | 3.4 a 5.4 g/dL |
| Total bilirubin | 0.81 mg/dL | 0.1 a 1.2 mg/dL |
| LDH | 642 U/L | 85-227 U/L |
| Deficiencies | ||
| Folic acid | 4.3 ng/mL | 2.7 a 17 ng/mL |
| Vitamin B12 | < 83 pg/mL | 200 a 800 pg/mL |
| 25-hydroxyvitamin D | 6.9 ng/mL | 30-40 ng/mL |
| Ferritin | 194 ng/mL | 23.9-336.2 ng/mL |
| Thyroid function | ||
| Free T4 | 1 ng/dL | 0.9 a 2.3 ng/dL |
| TSH | 0.39 mUI/L | 0.37 a 4.7 mUI/L |
| Others | ||
| Anti-tissue transglutaminase IgA antibodies | 1.41 |
Negative: less than 0.9 Undetermined: 0.9-1.1 Positive: greater than 1.1 |
RDW: red cell distribution width; AST: aspartate aminotransferase; ALT: alanine aminotransferase; IgA: immunoglobulin A; INR: international normalized ratio; LDH: lactate dehydrogenase; TSH: thyroid-stimulating hormone. Source: Author’s File.
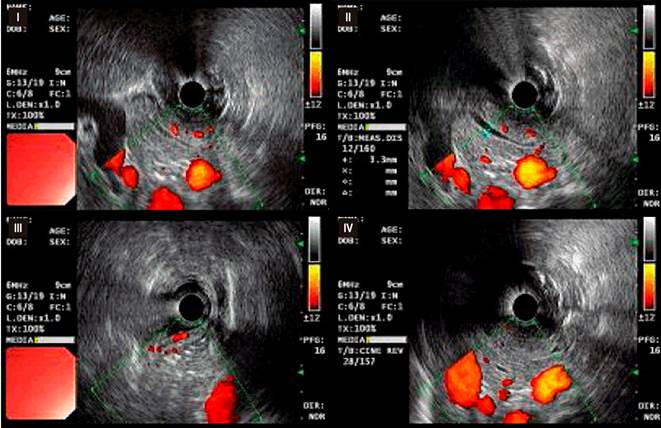
Based on the clinical and laboratory findings, celiac disease (CD) was suspected. Additional studies were conducted, including a duodenal biopsy following the protocol for CD, which reported findings consistent with Marsh 1 (Figure 1) and Marsh 2 (Figure 2) classifications, and an endoscopic ultrasound showing findings consistent with chronic pancreatitis (Figure 3). After discharge, the patient had positive anti-tissue transglutaminase IgA antibody results and normal IgA levels.
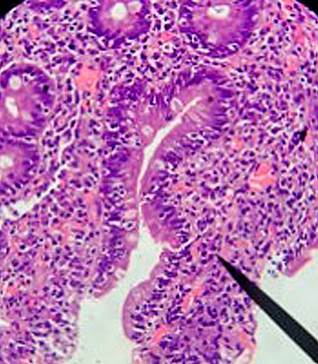
Figure 1 Marsh 1 Classification (Infiltrative). Duodenal bulb mucosa showing preservation of the glandular population with occasional intraepithelial lymphocytes (10 lymphocytes per 100 epithelial cells). Courtesy of the Pathology Department of Hospital Universitario San José, Popayán, with reading amplification (Dr. Nohelia Muñoz).
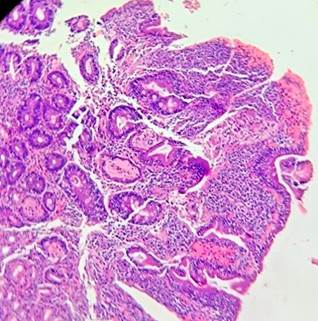
Figure 2 Marsh 2 Classification (Hyperplastic). Mucosa of the second portion of the duodenum showing slight glandular hyperplasia with 30 intraepithelial lymphocytes per 10 epithelial cells. There is no presence of villous atrophy. Courtesy of the Pathology Department of Hospital Universitario San José, Popayán, with reading amplification (Dr. Nohelia Muñoz).
Source: Author’s File.
Figure 3 Endoscopic Ultrasound. Pancreatic parenchyma showing changes in its echostructure (I), with the presence of hyperechoic bands between 3 and 5 mm (II) and diffuse nodularity throughout the gland (III). The duct of Wirsung is dilated, measuring approximately 3.5 mm towards the head, with wall reinforcement and irregularity (IV).
The patient was treated with a gluten-free diet (GFD), pancreatic enzyme supplementation, vitamin D, calcium, and monthly intramuscular vitamin B12. Following adherence to medical recommendations and a healthy lifestyle, the patient showed significant clinical improvement, gained 16 kg, normalized her bowel habits, and recent laboratory tests showed normal vitamin B12 levels and improved 25-hydroxyvitamin D levels (Table 2).
Table 2 Outpatient Laboratory Results
| Laboratory Test | Result | Reference Values |
|---|---|---|
| Vitamin B12 | 733.4 pg/mL | 200 a 1100 pg/mL |
| 25-hydroxyvitamin D | 17.7 ng/mL | 30-100 ng/mL |
Source: Author’s File.
A periodic follow-up plan at 3, 6, and 12 months was indicated, and recommendations were given for family members to undergo screening for the disease.
Discussion
Celiac disease (CD) is a systemic, immune-mediated disorder that occurs in genetically predisposed individuals in response to the ingestion of gluten, a protein found in wheat, rye, and barley6. More than 95% of patients with CD express HLA-DQ2, while the remaining patients express HLA-DQ8, along with other non-HLA genes7,8.
CD affects about 1% of the global population, with prevalence estimates ranging from 1.4% to 1.8%. There are significant regional differences and intra-regional variations, with cumulative incidences reaching up to 3%. These differences are not fully understood and cannot be explained by the known genetic and environmental risk factors1. The majority of cases present during childhood, with a higher prevalence in females. Risk factors include having a first-degree relative with CD, type 1 diabetes, Hashimoto’s thyroiditis, other autoimmune diseases (such as autoimmune liver diseases, Sjögren’s syndrome, and IgA nephropathy), Down syndrome, Turner syndrome, and IgA deficiency9.
In Colombia, there are no established protocols for the diagnosis or treatment of CD, leading to low suspicion and underreporting of the disease. This may also be attributed to lower gluten consumption compared to industrialized countries, variations in gut microbiota, or early childhood exposure to infections that might prevent the development of autoimmunity10.
The classic clinical presentation of CD includes chronic diarrhea, abdominal distension, and weight loss, as seen in the patient under discussion. Other common symptoms include recurrent abdominal pain, constipation, chronic fatigue, headache, aphthous stomatitis, dermatitis herpetiformis, osteoporosis, iron deficiency, anemia, elevated transaminases, and neurological symptoms such as anxiety, depression, and paresthesias, the latter being present in the described patient. Up to 21% of patients are asymptomatic. This patient presented with severe anemia, deficiencies in vitamins B12 and D, and possibly vitamin K deficiency (suggested by the prolonged INR). These findings, along with the clinical symptoms, indicated a malabsorption syndrome, leading to the suspicion of CD1,9.
Patients with CD are at an increased risk of developing acute or chronic pancreatitis, which can worsen their overall prognosis. The prevalence of CD in patients with chronic pancreatitis can be as high as 7.4%, as seen in the case of the patient described. Several theories have been proposed to explain this increased risk: malnutrition associated with CD affecting pancreatic secretion and causing atrophy; chronic inflammation of the duodenum and the papilla area leading to stenosis and subsequent pancreatic disease; and an autoimmune phenomenon involving specific autoantibodies against pancreatic islets, which is considered one of the most significant contributors to the relationship between pancreatitis and CD. Both endocrine and exocrine pancreatic functions can be affected, with exocrine insufficiency being more common in CD patients due to reduced secretin production resulting from duodenal involvement. This exacerbates the patients’ nutritional status and prognosis11.
The gold standard for diagnosing CD is a combination of positive serological tests-anti-tissue transglutaminase antibodies (tTGA-IgA), anti-endomysial antibodies, and antibodies against deamidated gliadin peptides-along with a duodenal biopsy following the CD protocol8,12. It is noteworthy that up to 80% of individuals with CD remain undiagnosed, as the prevalence of biopsy-diagnosed CD is approximately 0.7%1. In the patient described, positive serological tests and duodenal biopsy findings classified as Marsh 1 and 2 confirmed the diagnosis of CD13.
The severity of histological changes in the small intestine does not necessarily correlate with the severity of clinical manifestations. In 2007, Brar and colleagues studied 499 CD patients and demonstrated a trend towards a higher proportion of patients presenting with atypical or silent (non-diarrheal) CD. This was accompanied by a trend towards less severe duodenal pathology; however, most patients still had the more severe form of villous atrophy, indicating a lack of correlation between clinical presentation and the degree of villous atrophy14. This was further evidenced in a 2013 Italian study, which included histological data from over 1400 adult CD patients and found that patients with mild enteropathy could not be distinguished from those with villous atrophy based on laboratory characteristics, with the possible exception of the t-TG antibody index15.
The management of CD relies on strict adherence to a GFD4. Symptom improvement and resolution typically occur within days or weeks and often precede the normalization of serological markers and the atrophy of duodenal villi1. All patients with CD should be tested for micronutrient deficiencies, including iron, folic acid, vitamin B12, and vitamin D, to enable timely supplementation if deficiencies are identified. Additionally, treatment with digestive enzymes is essential while managing CD with the GFD11.
Periodic follow-up and monitoring of CD patients are recommended to assess the response to treatment, detect associated diseases, and monitor the progression to refractory forms of CD or neoplastic complications arising from the condition.
Conclusion
Celiac disease is a systemic, immune-mediated, and multifactorial pathology with a rising prevalence due to increased gluten consumption in the diet and greater clinical awareness for its diagnosis. It can affect individuals of any age and manifests predominantly in children and women. CD presents a diagnostic challenge, requiring high clinical suspicion and physician awareness to perform timely serological and histological studies for early management, thus significantly impacting patients’ quality of life.
It is crucial to suspect CD in patients with compatible gastrointestinal symptoms, particularly those that are recurrent or refractory to conventional therapy, as well as in patients with chronic, resistant, or recurrent idiopathic pancreatitis.











 text in
text in 



