Introduction
Corn is one of the bases of planetary food security. It is the second most important crop after wheat (Paliwal, 2016). Colombia is the fourth largest corn producer in South America, and this crop is the third most sown in the country (CIAT & CIMMYT, 2019). Simijaca is a regional corn variety of free pollination. It is adapted to cold climate production zones in Colombia. This variety was widely cultivated by the Muisca, the indigenous inhabitants of the Ubaté valley region who selected it for its culinary quality. Therefore, this corn variety has been highly accepted in Andean cities (Ligarreto, 2017). In recent years, corn crops in the municipality of Simijaca at the Ubaté valley have been affected by stalk rot but without accurate diagnosis (Gómez et al., 2017). This is a complex disease and its cause is difficult to determine since many fungi, bacteria, and oomycetes may appear in affected plants as secondary invaders and saprophytes (Wicklow et al., 2005).
Stalk rot caused by Fusarium spp. typically reduces yield by 10% or 30%-50% in severely affected areas (Gai et al., 2018). Although Fusarium stalk rot (FSR) is among the most economically important diseases of corn around the world (Yang et al., 2010; Wang et al., 2017), it is not frequently seen in Colombia and had not been reported in Cundinamarca until now (Buritica, 1999). In production areas around the world, at least 22 Fusarium species can be found in corn causing diseases (Munkvold et al., 2018). Two species, Fusarium graminearum in the Fusarium graminearum species complex (FGSC) and Fusarium verticillioides within the Fusarium fujikuroi species complex (FFSC) are the main causes of FSR in corn. The first species is more common in cold regions and is one of the most damaging causal agents of stalk rot, while the second Fusarium species is more common in hot, dry climates and is particularly damaging if it attacks before flowering (CIMMYT, 2004). In Colombia, the ancient F. fujikuroi has been reported causing pink rot in seeds and ears in Antioquia, Cordoba, Cundinamarca, Santander, Tolima, and Valle del Cauca, whereas F. graminearum has been reported as associated with stalk and ear rot in Antioquia, Cordoba, Nariño, and Valle del Cauca (Buritica, 1999).
Fusarium sambucinum species complex (FSAMSC) includes the FGSC, F. cerealis, and F. culmorum (Laraba et al., 2021). Several taxa have been identified within the FGSC, and attempts have been made to divide this complex into phylogenetically separated species. Some of them are present in particular continents. However, it is arguable whether these taxa should all be defined as species, or if they reflect populations or lineages within a broader concept of F. graminearum sensu lato (Summerell, 2019). In the last 30 years, reviews of FFSC have reported 45 phylogenetic species, 10 biological species and 34 morphospecies. This complicates the identification of new isolates based only on morphological characters, generating misclassification and underestimation of species diversity (Leyva-Madrigal et al., 2015). The taxonomy within FFSC and FGSC is mainly based on DNA sequence analysis of calmodulin, elongation factor 1-α (EF1), and β-tubulin genes identifying most of the species in these complexes (Leyva-Madrigal et al., 2015). Currently EF1 is the most widely used molecular marker in phylogenetic and taxonomic studies within the Fusarium genus (Stakheev et al., 2018).
Fusarium spp. produce different kinds of spores that may be transported and disseminated by air, raindrops, insects and seeds and that are infected through the pistils (Windels et al., 1976; Ooka & Kommedahl, 1977; Munkvold et al., 1997; Duncan & Howard, 2010). F. graminearum, F. verticillioides, and Fusarium proliferatum (the last two in the FFSC) enter the corn plant through trichomes, leaves, xylem and stems (Nguyen et al., 2015; Nguyen et al., 2016). There is evidence of Fusarium spp. endophytes in wild and cultivated plants and the best example is F. verticillioides. In this case, F. verticillioides is associated with corn plants along the complete crop cycle, where plant responses to the infection depend on several factors related to plants, fungi, and the environment (Kuldau & Yates, 2000). This ancient and evolutive relationship can promote plant growth, protect the seed from infection by other 10 genera of fungi while the plant serves as a source of carbon and a pathway of vertical and horizontal transmission of the fungus (Van Wyck et al., 1988; Wicklow, 1988; Yates et al., 1997; Schulz et al., 1999; Kuldau & Yates, 2000).
The endophyte state is transient and F. verticillioides switches from an asymptomatic and biotrophic lifestyle to an hemibiotrophic one causing disease (Schulz et al., 1999). Stress conditions promote the disease onset and a range of virulence can be observed among different strains. However, strains that can be pathogenic are known to be asymptomatic under optimal plant growth conditions (Kuldau & Yates, 2000). Thermal stress (cold and heat), drought, high sowing density, shadow, pest attacks and the use of fertilizers with high nitrogen and low potassium content are examples of stress conditions that can promote diseases on a very well-balanced association between the plant and F. verticillioides (Dodd, 1980; Schulz et al., 1999; Kuldau & Yates, 2000; Blandino et al., 2009).
Given the recent problem of corn lodging caused by stalk rot in high-altitude corn-producing regions of the Ubaté valley of Colombia, the objectives of this study were to determine the causal agents of corn lodging associated with stalk rot in this corn-producing region and describe the symptoms of the disease. Our research describes the symptoms and signs associated with stalk rot in corn plants of the regional varieties Simijaca and Sogamoso (var. Simijca and var. Sogamoso) under field conditions. The associated causal agents were morphologically and molecularly identified, and their pathogenicity was determined in corn plants of the regional variety Simijaca (var. Simijaca).
Materials and methods
Description of symptoms and signs
During 2016, corn seeds of the regional varieties Simijaca and Sogamoso were sown (22000 plants ha-1) in two plots with stalk rot reports located in the municipality of Simijaca (Cundinamarca, Colombia) (5°29'49"N; 73°49'55"W and 5°33'11"N; 73° 47'29"W). These plots were selected due to the fact that the disease has been reported since 2016 and symptoms of stalk rot have been observed in corn crop cycles during recent epidemics in the region. Plants were randomly inspected on a monthly basis from sowing to tasseling to describe disease symptoms and signs of the pathogen. The inspection dates matched the developmental stages of three, six, nine true leaves, tasseling, silking, and grain with 40% of dry weight (V3, V6, V9, VT, R1, and R4, respectively) according to the scale proposed by Hanway et al. (1966). Diseased and healthy plants of both corn varieties in each development stage of the crop were collected and transported to the laboratory of plant pathology (Universidad Nacional de Colombia, Bogotá campus) for detailed inspection under stereoscope, processing and pathogen isolation to identify the causal agent of the disease. Data of precipitation (mm), relative humidity (%), wind speed (m s-1), maximum, minimum and average temperatures (°C) were registered using an iMETOS® 300 climatic station (Pessl instruments, Weiz, Austria) at a 10 min frequency. Data were registered in the two experimental plots and inspected in real time. At the end of the trials, the climatic data obtained were compared with a 30-year database (1986-2016) for the municipality of Simijaca provided by the Corporación Autónoma Regional de Cundinamarca (CAR).
Fusarium isolation from corn plants affected by stalk rot
Fusarium spp. was isolated from symptomatic plants following the Murillo-Williams and Munkvold (2008) protocol. For this purpose, tissue from roots, crown and stalk was collected, disinfected, and sown in Petri dishes with potato dextrose agar (PDA) medium (Oxoid®) acidified at 0.1% (v/v) with lactic acid. The dishes were incubated under dark conditions at 25°C for 10 d (Model FD 23, Binder®, Germany). Afterwards, the frequency of Fusarium isolation per corn variety and the plant's explants origin were recorded. The most representative Fusarium colonies were purified in PDA and monosporic cultures were obtained in 3% agar (30 g L-1) (WA) (Oxoid®) amended with 12 ml L-1 chloramphenicol and 20 ml L-1 streptomycin sulfate (Leslie & Summerell, 2006). These cultures were then incubated on PDA at 25°C with a 12:12 h light/dark photoperiod for 15 d in growth chambers (MLR- 351H, Sanyo®, Japan). The resulting pure isolates were stored at -70°C in 15% glycerol. Fusarium frequencies, according to the corn variety and part of the plant used for isolation of the pathogen, were analyzed under a completely randomized design (n=24) and subjected to normality and variance tests; means were compared using the Tukey's test (P=0.05).
Morphological identification of Fusarium spp.
Morphological identification of the produced Fusarium isolates was performed according to Leslie and Summerell (2006). Carnation leaf piece agar (CLA), Spezieller Náhrstoffarmer Agar (SNA), WA and PDA media were used and incubated in growth chambers (MLR- 351H, Sanyo®, Japan) at 25°C with a 12:12 h light/dark photoperiod for 15 d. The color of sporodochia, shape and size of macroconidia and microconidia, number of septa, type of conidiogenesis, formation of chlamydospores and perithecia were determined by light microscopy (CX 31, Olympus®, Japan). Additionally, pigmentation, appearance of the colony, and rate of mycelial growth were evaluated in PDA medium under the same incubation conditions previously described.
Molecular identification of Fusarium spp.
Molecular identification was performed by sequencing the elongation factor 1-α (EF1) following the methodology of Stakheev et al. (2018). For this purpose, 100 mg samples of fresh mycelium per Fusarium isolate were taken from seven-day-old colonies grown on PDA and mechanically lysed with 3 mm diameter tungsten beads in a TissueLyser (Qiagen®, Hilden, Germany) (30 Hz/5 min). DNA extraction was performed using the Plant/Fungi DNA Isolation Kit (Norgen Biotek Corporation®, Canada) following the manufacturer recommendations. Species were identified using the polymerase chain reaction (PCR) of the elongation factor 1-α (EF1) using the oligonucleotides EF50Fw: 5' CGACTCTGGCAAGTCGACCAC 3' and EF590R: 5' CTCGGCTTTGAGCTTGTCAAG 3' following the methodology of Stakheev et al. (2018). The phylogenetic analysis was performed using the CLASSIFIER algorithm from the package MEGA version 7.0 (MacOS), using the neighbor-joining methodology with 1000 bootstrap replicates. The phylogenetic tree for EF1 was built using the T92+G model described by Tamura (1992).
Pathogenicity test of Fusarium graminearum and Fusarium subglutinans on corn regional variety Simijaca and their effect on plant growth
For the pathogenicity test, F. graminearum (26B) was multiplied following the chaff-grain methodology (Leslie & Summerell, 2006) and F. subglutinans (45D) was multiplied in liquid Czapek medium while stirring (Inkubator 1000 - Unimax 1010, Heidolph, Germany) at 150 rpm for 15 d at room temperature (± 20°C) (Leslie & Summerell, 2006). Conidia of each Fusarium species were harvested, centrifuged at 2500 rpm for 15 min (MIKRO22R, Hettich® UK), and the inoculum suspensions were adjusted to 1.0 x 10-1 conidia ml-1 by hemocytometer counting (Neubauer, VWR, Darmstadt, Germany).
Seeds of the regional variety Simijaca were used and treated with hot water at 52°C for 5 min in an evaporator (Water B-480, BÜCHI Labortechnik®, AG, Switzerland) following the methodology of Daniels (1983). Seeds were then inoculated with 100 ml of the previously prepared conidia suspension of F. subglutinans and F. graminearum by shaking the mixture vigorously (Wilke et al., 2007). Additionally, the combined inoculation with both F. subglutinans and F. graminearum was conducted using 50 ml of a suspension of each species. The inoculation of the Fusarium species obtained (F. subglutinans, F. graminearum, and the mixture) was evaluated and considered as treatments (Reid et al., 1999). Seeds without treatment and seeds treated with heat were used as absolute control and thermal control, respectively, and were mock inoculated with sterile distilled water (SDW).
After inoculation, the seeds were placed on plastic trays with a 5 cm layer of soil (Warham et al., 1997) from a non-agricultural area from which the presence of Fusarium was previously ruled out according to Leslie and Summerell (2006). Once the seeds germinated, 40 seedlings were selected per treatment. These seedlings were then individually transferred to bags with 1.5 kg of soil of the same origin and taken to a greenhouse (± 25°C, ~75% relative humidity). Three months after sowing (V9), longitudinal cuts of plant stems were taken to evaluate the presence of symptoms associated with stalk rot. Plants showing apical chlorosis, leaf anthocyanosis, and dwarfism were considered diseased plants. Incidence of the disease and internal rot stalk (I) were determined using Equation 1 according to Madden et al. (2007).
where Pd represents the number of plants showing the characteristic, and Pt is the total number of plants per treatment. Pathogen isolation was conducted 90 d after sowing (DAS) on PDA medium at 25°C as described above. Plant height (cm) from the stem base until the tip of the third true leaf and stem diameter at the base (mm) were also registered. Data analysis was conducted using a completely randomized design (n=40) and subjected to normality and variance tests; mean comparisons between treatments were performed using the Tukey's test (P= 0.05).
Results
Symptoms of stalk rot (FSR) and signs of the pathogen
The external symptoms of FSR in plant grown in the municipality of Simijaca at the Ubaté valley corresponded to apical chlorosis, leaf anthocyanosis, and plant dwarfism (Fig. 1A-C). Intense necrosis of the crown and plant nodes was detected in longitudinal stalk sections, and progressed towards the internodes (Fig. 1G) causing basal disintegration (Fig. 1I).

FIGURE 1 External and internal symptoms of stalk rot In corn plants of the regional variety Simijaca three to five months after planting (V6-R1) in the municipality of Simijaca (Cundinamarca, Colombia). A) Apical chlorosis of leaves (V9), B) leaf anthocyanosis (V9), C) plant dwarfism (V9), D) plant lodging (R1), E) healthy plant (V9), F) dissemination of the disease through nodes (V6), G) crown and node necrosis (VT), H) progress of the lesion from nodes to internodes (VT), and I) rot and stalk base disintegration (R1).
In longitudinal sections of healthy plants, the pith was cream-colored and the pith of diseased plants had occasional purple coloration (Fig. 1H-I). Lodging, as the final manifestation of corn stalk rot, occurred at any phenological stages of the crop (Fig. 1D). Initial infection was contained in the crown of the plant and then spread to all the plant levels through the nodes colonized by the pathogen at initial stages of plant development, causing a systemic infection (Fig. 1F).
Fusarium isolation and morphological identification
Although there was no statistical difference of Fusarium frequency of isolation between corn varieties (P=0.091) or between the different plant organs analyzed (P=0.112), isolation was 44%, 58%, 78%, and 87% for developmental stages of three, six, and nine true leaves and tasseling, respectively (V3, V6, V9, and VT). Progressive colonization of the stem was observed, with Fusarium spp. detected at low frequencies at V3 increasing at V6, V9, and VT. Similar frequencies were observed on the crown and roots (data not shown). Two representative morphotypes of Fusarium were isolated from symptomatic plants, purified and morphologically identified.
F. graminearum (isolate 26B) showed white colonies that changed to ocher and reddish tones with a feathery appearance (Fig. 2A) on PDA medium. Brown sporodochia were observed on CLA medium (Fig. 2B). Macroconidia were slightly swollen in the middle, 40-50 x 4.5-5.5 in size with five or six septa moderately curved, with the ventral side straight and the dorsal side arched. The basal cell was foot-shaped, and the apical cell straight with a narrow hook or beak (Fig. 2C). Superficial perithecia of F. graminearum were observed on the crop debris of the regional corn varieties Simijaca and Sogamoso, mainly on the stalk nodes of diseased plants. These structures were red under lactophenol blue staining with asci containing usually eight trisected-ascospores of approximately 38 in length (Fig. 2D-E).
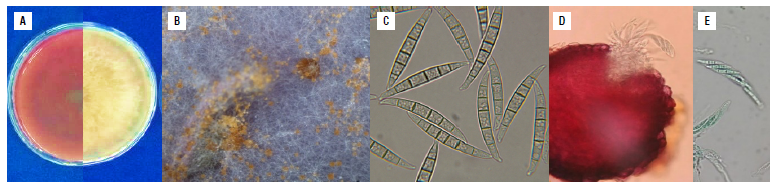
FIGURE 2 Morphological characteristics of Fusarium graminearum isolate 26B (in the Fusarium graminearum species complex FGSC), isolated from co rn plants with stalk rot. A) Characteristics of the colonies, right: appearance of the colony and left: pigmentation on the back of the Petri dish on PDA medium 15 d after culture, B) brown sporodochia on CLA medium, C) macroconidia on CLA medium, slightly swollen in the middle, with five or six septa, moderately curved, with the ventral side straight and the dorsal side arched. The basal cell was foot-shaped, and the apical cell straight with a narrow hook or beak, D) In situ perithecia on CLA medium, E) asci and ascospores. PDA - Potato Dextrose Agar; CLA - Carnation Leaf Agar.
Fusarium subglutinans (isolate 45D) showed white cottony colonies with orange and purple colorations (Fig. 3A) on PDA medium. On CLA medium, orange sporodochia were formed (Fig. 2B) and macroconidia were typical of the FFSC, 50-60 x 3-4 in size, three to four septa, straight with curved apical cell and poorly developed basal cell. Microconidia were predominantly oval, usually without septa and (Fig. 3C) forming pseudo-heads over polyphialids (Fig. 3D). Chlamydospores were not formed on CLA, WA or SNA media. On PDA medium, the growth rate of F. subglutinans was less than 1.0 cm per day, whereas F. graminearum showed a growth rate over this value.

FIGURE 3 Morphological characteristics of Fusarium subglutinans Isolate 45D (In the Fusarium fujikuroi species complex FFSC), isolated from corn plants with stalk rot. A) Characteristics of the colonies, appearance of the colony (right) and pigmentation on the back of the Petri dish on PDA medium 15 d after culture (left), B) orange sporodochia on CLA medium, C) macroconidia typical of the fujikuroi complex with three to four septa, straight with curved apical cell and poorly developed basal cell and microconidia predominantly oval, usually without septa, D) microconidia in situ on CLA medium forming pseudoheads. PDA - Potato Dextrose Agar; CLA - Carnation Leaf Agar.
Molecular identification of Fusarium spp. from corn plants with stalk rot
The phylogenetic trees of EF1 is shown in Figures 4 and 5 in which isolate 26B (MT598159) was grouped with the graminearum clade containing F. culmorum, F. cerealis and two species belonging to the FGSC (F. graminearum sensu stricto and F. ussurianum) (72%) within the FSAMSC (Fig. 4). Isolate 45D (MT598158) was grouped with species of the FFSC (98%) and the species F. subglutinans (100%) (Fig. 5). Finally, EF1 sequences were annotated in the National Center for Biotechnology Information (NCBI).
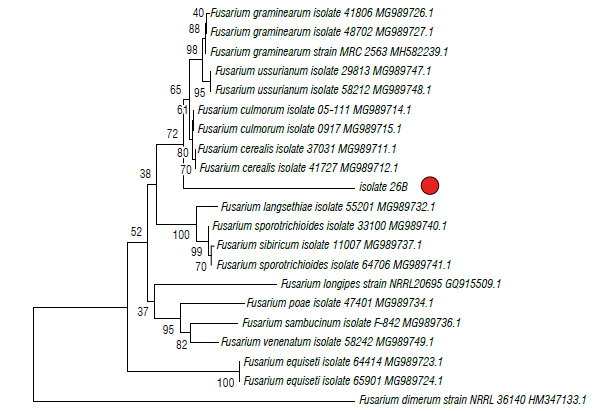
FIGURE 4 Phylogenetic tree of fungal Isolates 26B obtained from corn plants with stalk rot symptoms and obtained by the Elongation factor 1-α (EF1). The evolutionary history was Inferred using the neighbor-joining method. The percentage of replicate trees in which the associated taxa clustered together in the bootstrap test (1000 replicates) Is shown next to the branches. The tree is drawn to scale, with branch lengths in the same units as those of the evolutionary distances used (0.05) to Infer the phylogenetic tree. The evolutionary distances were computed using the Tamura 3-parameter method and are in the units of the number of base substitutions per site. The rate variation among sites was modeled with a gamma distribution (shape parameter=3).
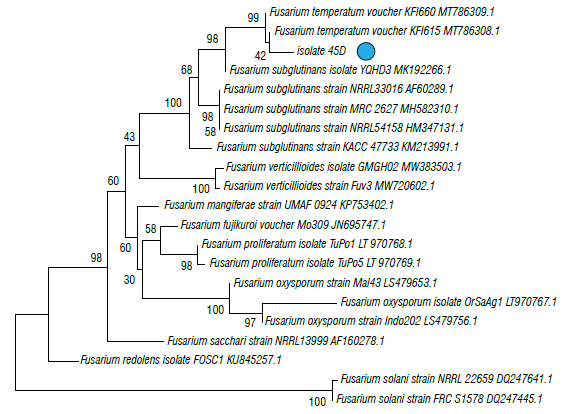
FIGURE 5 Phylogenetic tree of fungal isolates 45D obtained from corn plants with stalk rot symptoms and obtained by the Elongation factor 1-α (EF1). The evolutionary history was inferred using the neighbor-joining method. The percentage of replicate trees in which the associated taxa clustered together in the bootstrap test (1000 replicates) Is shown next to the branches. The tree is drawn to scale, with branch lengths in the same units as those of the evolutionary distances (0.05) used to Infer the phylogenetic tree. The evolutionary distances were computed using the Tamura 3-parameter method and are in the units of the number of base substitutions per site. The rate variation among sites was modeled with a gamma distribution (shape parameter=3).
Pathogenicity of Fusarium graminearum and Fusarium subglutinans on corn regional variety Simijaca and their effect on plant growth
Isolates 26B of F. graminearum and 45D of F. subglutinans were pathogenic in corn plants of the regional variety Simijaca. At 90 d after sowing (DAS), the inoculated plants showed necrosis and reddish to purple colorations in the basal internal part of the stalk (Fig. 6). Incidence of internal symptoms was observed in 40% of the plants inoculated with F. graminearum and 25% of plants inoculated with F. subglutinans, whereas these symptoms were observed in 20% of plants inoculated with both species. Fusarium graminearum showed a tendency to be located mainly in plant nodes (Fig. 6B), whereas F. subglutinans was located in the internodes (Fig. 6C). Necrosis of nodes and internodes occurred with the inoculation of the combination of both Fusarium species (Fig. 6A). The thermal control did not show symptoms (Fig. 6E), and absolute control showed a low degree of affectation (Fig. 6D). From their respective treatment, F. subglutinans and F. graminearum were isolated on PDA medium and morphologically identified fulfilling Koch postulates. Fusarium was not isolated from the thermal control.

FIGURE 6 Pathogenicity tests of Fusarium spp. in corn variety Simijaca three months after sowing (V9) under greenhouse conditions. A) Longitudinal sections of plants inoculated with the mixture of F. subglutinans and F. graminearum, B) F. graminearum, C) F. subglutinans, D) absolute control (seeds without treatment), and E) thermal control (seeds treated with hot water at 52°C).
Regarding plant height, the individual inoculation of F. subglutinans and F. graminearum had a significant effect generating the tallest plants (98 cm) (P<0.0001) at 90 DAS, whereas the joint inoculation of Fusarium species and the absolute control (seeds without thermal treatment) had the shortest plants (90 cm), and intermediate values were obtained on the thermal control (P<0.0001). Although stem diameter data were not adjusted to normality, the joint inoculation of F. subglutinans and F. graminearum suggests a higher stem diameter compared to the other evaluated treatments.
Discussion
Pathogenicity tests carried out in corn seeds of the regional variety Simijaca developed symptoms similar to those initially observed in the field after inoculation of isolates 26B of F. graminearum and 45D of F. subgluti-nans. Fusarium graminearum appears to cause necrosis mainly in the nodes and crown of the plant, whereas F. subglutinans caused necrosis in the pith and crown. In this study, the joint inoculation of both Fusarium species caused symptoms in the crown, nodes and internodes. Therefore, the obtained results may be considered as the first report of F. graminearum in the FGSC and F. sub-glutinans within the FFSC causing corn stalk rot in corn crops in Cundinamarca (Colombia), specifically in the municipality of Simijaca. Internal symptoms observed in the pathogenicity tests conducted with thermal treated seeds showed the positive effect of the treatment on the reduction of symptoms. The effect of the treatment was clear in plants of thermal control (seeds treated with hot water at 52°C) that showed the normal cream-white color of the inner stem. In contrast, an internal purple color of the pith of plants was observed in plants of the absolute control (seeds without treatment). This is according to the report for contaminated seeds, as natural source of Fusarium spp. inoculum (Duncan & Howard, 2010). These observations are also in contrast to the more severe symptoms observed in the field study, that used untreated corn seeds. Similar positive results have been reported for the thermal treatment of corn seeds and other cereals (Clear et al., 2002; Coutinho et al., 2007; Bennett & Colyer, 2010; Piñeros-Guerrero et al., 2019). The lack of continuity of the necrotic area in nodes and internodes can be explained by the morphology and development of corn plants, which is used by Fusarium spp. for its plant colonization and dissemination. The nodes formed by the apical meristem are initially contained in the crown of the plant (Nielsen, 2008). Once the stem elongation begins, these nodes may cause a systemic infection of the plant if infected by the pathogen, as observed in this study. This is consistent with the histological observations conducted by Lawrence et al. (1981), who find that the fungus initially confined to the basal parts of the stalk had a rapid spread along the plant at the time of flowering.
The symptoms and signs found in this study were similar to those reported for stalk rot caused by Fusarium spp. in corn (CIMMYT, 2004). Additionally, superficial perithecia of F. graminearum were found in crop debris, mainly in stalk nodes. Unlike F. graminearum which is a homothallic fungus (Leslie & Summerell, 2006), F. subglutinans and F. verticillioides are heterothallic. This characteristic explains why perithecia of F. verticillioides are rarely observed in nature, although they are easily inducted under laboratory conditions. The same may occur with F. sub-glutinans.Blacutt et al. (2018) stated that in contrast to F. graminearum, where ascospores are the primary inoculum source, sexual reproduction in F. verticillioides and other species within FFSC contributes to their genetic diversity without being essential for their life cycle.
The Fusarium species associated with Simijaca and Sogamoso corn plants were morphologically and phyloge-netically identified as F. graminearum in the FGSC and F. subglutinans within the FFSC that are reported worldwide as causal agents of stalk rot in corn (CIMMYT, 2004; Leslie & Summerell, 2006). The lineages defined as FGSC are morphologically indistinguishable (Yli-Mattila et al., 2009; Summerell, 2019). In this study, isolate 26B, morphologically and biologically identified as F. graminearum, was phylogenetically grouped with F. culmorum, F. cerealis and two species belonging to the FGSC (F. graminearum s.s. and F. ussurianum) within the FSAMSC. The Fusarium species described in this study, probably, belong to the ho-mothallic species F. graminearum and not to F. cerealis or F. culmorum. This is supported by the fact that superficial perithecia were found on the nodes of the stalk of corn plants, and the sexual stage of F. cerealis and F. culmorum is unknown (Leslie & Summerell, 2006).
Fusarium boothii (in the FGSC) with the 15-acetyldeoxyni-valenol (15 ADON) chemotype and Fusarium meridionale (in the FGSC) with the nivalenol (NIV) chemotypes are the lineages/species/chemotypes endemic to South America. However, F. asiaticum (in the FGSC) with its 3-acetylde-oxynivalenol (3-ADON) and NIV chemotypes has been introduced in the region. In general, F. graminearum s.s. 15 ADON is the most common species in Brazil and Argentina with some displacement by more aggressive 3 ADON populations (Van der Lee et al., 2018). Determining the species/lineages and chemotypes to which isolate 26B belongs may contribute to the knowledge of species/ lineage distribution in the cold tropics of Colombia in South America. Additionally, these results may help to predict toxicological risks and aggressiveness according to the species/lineages and chemotypes present.
Isolate 45D, identified in this study as F. subglutinans, may represent two cryptic species distinguishable by amplified fragment length polymorphism (AFLP): F. subglutinans s.s. and F. temperatum in the FFSC (Czembor et al., 2015; Fumero et al., 2016). Determining the species to which this isolate belongs is important to predict its distribution and toxicological profile since F. temperatum apparently produces fumonisins (Wang et al., 2014), beauvericins and fusaproliferin. Fusarium subglutinans does not produce fumonisins, but it does produce other types of mycotoxins such as moniloformines and fusaproliferin (Fumero et al., 2016). F. subglutinans is frequent in cold areas of Peru, Mexico, and Argentina (Logrieco et al., 1993; Figueroa-Rivera et al., 2010; Reyes-Velázquez et al., 2011; Fumero et al., 2016) and F. temperatum has been found in Argentina and Southern Brazil (Fumero et al., 2016). Future studies should be carried out to document the species occurrence within the FFSC in the Colombian cold tropics of South America, where F. verticillioides is the most common species in warm parts of the continent (Chulze et al., 1996).
In this research, we found F. subglutinans in the FFSC and F. graminearum within the FGSC associated with the corn stalk rot disease on the regional varieties Simijaca and Sogamoso, which are adapted to the cold and high altitude production zones in Colombia. This result matches the effect of latitude and altitude on the distribution of Fusarium species reported by Munkvold et al. (2018) in corn. These authors observe that F. verticillioides prevails in warm and dry tropical and subtropical areas, whereas F. graminearum and F. subglutinans are the dominant species in cold-temperate regions as altitude increases, with reports in Europe, Asia, Oceania, and North and South America.
Although Fusarium stalk rot is among the most economically important diseases of corn around the world, it had not been reported in the country for at least 30 years. Therefore, corn stalk rot may be considered an emergent disease in Colombia according to our findings and the epidemics occurring in corn plots in Simijaca at the Ubaté valley in the last years. The climatic conditions observed during the period of the study (2016 and 2017) were colder, with average temperatures of 13°C (below the historical average of 14°C). Additionally, dry conditions framed on a tropical El Niño episode with accumulated precipitation of429 mm (125 mm lower than the historical) were also registered for this period of time in the Ubaté valley. Therefore, cold stress and drought conditions could have contributed to a shift in the biotrophic and symptomless association between the Simijaca corn variety and the Fusarium spp. migrating toward a disease-causing condition (stalk rot) (Dodd, 1980; Schulz et al., 1999; Kuldau & Yates, 2000; Bacon et al., 2008; Blandino et al., 2009). Because of their importance, the findings of this study need to be expanded. Therefore, further studies should be conducted with a higher number of isolates and the potential of toxin production of the species present in the field should be evaluated. Further research regarding Fusarium species diversity in corn along the Andean region in the Colombian cold tropic and a more robust phylogenetic analysis must be conducted to determine the species/lineages present in the country. Evaluating the toxicological profile and aggressiveness of these species/ lineages may also contribute to an understanding of FSR in corn crops under cold climate conditions.
Conclusions
Fusarium graminearum within FGSC and Fusarium sub-glutinans within the FFSC were found associated with corn stalk rot in the Ubaté valley throughout the entire crop cycle, and its pathogenicity was confirmed in the corn variety Simijaca. The initial infection of the pathogen was contained in the crown, but it spreads towards the upper part of the plant through the nodes previously colonized at initial stages of plant development, causing a systemic infection. Necrosis of the crown, nodes and internodes and the pith showing purple colors could be observed in longitudinal stalk sections. These symptoms caused basal disintegration and lodging, as the final manifestation of the corn stalk rot that may occur at any phenological stage of the crop. Although Fusarium stalk rot is well reported as an economically important disease in corn, it could be considered an emergent disease under conditions of the Ubaté valley in Colombia.















