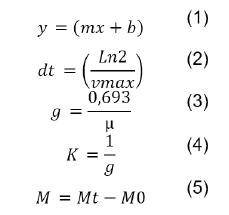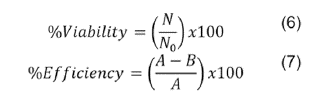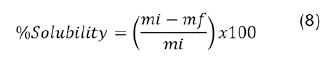Introduction
Food safety issues arise from food contamination, which can stem from various sources found in the environment, such as water, dust, soil, insects, and feces from mammals, birds, and reptiles1. These contaminants have the potential to impact agricultural production, food processing, and food preparation processes, giving rise to Foodborne Diseases (FBD), it is important to note that all types of food, including vegetables, fruits, meat, milk and its products, sausages, fish, and ready-to-eat foods, can serve as potential vehicles for the transmission of FBD2.
The World Health Organization (WHO) reports that 420 000 people die annually from FDB, which is caused mainly by Gram-negative bacteria and Gram-positive bacteria3. By 2020 in Europe, there were 3166 outbreaks of FDB, causing 22 010 illnesses, 1838 hospitalizations and 48 deaths, in the United States for the same time period there were 299 outbreaks, causing 5987 illnesses, 641 hospitalizations and fourteen deaths4. By 2020 in Colombia, 64,5 % (312/483) of the FBD outbreaks were sampled and 35,2 % (110/312) were identified by etiological agent, mainly Salmonella spp, Escherichia coli, Staphylococcus aureus, fecal coliforms, and total coliforms, and the symptoms presented in outbreaks of bacterial etiology were diarrhea (92,9 %), cramps (59,5 %), nausea and emesis (45,2 %), abdominal pain (16,7 %), headache (16,7 %), bloody diarrhea (14,3 %), fever (9,5 %), among others (10 %)5.
A FBD outbreak is identified when there are two or more cases with similar symptoms and is caused by the consumption of a common food6. Once contaminated food with FDB is ingested, elderly people, children, and pregnant women experience more severe gastrointestinal and clinical symptoms such as nausea, vomiting, diarrhea, abdominal cramps, joint pain or back pain, and fatigue7. It is important to mention that biological factors related to FDB exist as saprophytic or ubiquitous microorganisms, in general, the bacteria involved are S. aureus, E. coli, Salmonella and Listeria monocytogenes8. Authors mention the evolutionary development of these microorganisms better adapted to higher stress scenarios, one of the most important is Antimicrobial Resistance (AMR), especially S. aureus resistant to metacilin (SARM) resistant to beta-lactam antibiotics and only sensitive to fifth generation cephalosporins9.
S. aureus FBD is characterized by being an important cause of systemic infections, being the microorganism with the highest morbidity and mortality, a growing public health problem10. In Colombia, S. aureus is one of the main bacteria causing intramammary infections in dairy cattle, in these productions there is close contact between milkers, cattle and the zoonotic potential of S. aureus that represents a FBD11. In addition, about 40 % of raw milk is still produced under conditions of low safety, which can spread this pathogen in the general population12.
The Lactic Acid Bacteria (LAB) strains Pediococcus pentosaceus, Enterococcus faecium, Leuconostoc mesenteroides subsp. mesenteroides and Lactobacillus casei, were classified as having high inhibitory capacity against E. coli, L. monocytogenes, S. aureus. and P. pentosaceus13. Research shows that strains of Lactobacillus genus, such as L. casei, release peptides with high radical-binding and antimutagenic activity, which have demonstrated good response in animal feeding and efficacy in treatments for Helicobacter pylori infections and inhibition of pathogenic bacteria such as L. monocytogenes, E. coli O157:H7, Salmonella spp., S. aureus, and SARM in in-vitro tests14,15. L. casei is a well-researched species due to its commercial, industrial, and health potential. It is commonly used to ferment dairy products, resulting in foods with enhanced taste and texture. Additionally, it has been found to produce numerous bioactive metabolites that can provide health benefits to the consumer15,16. Lactobacillus bacteria offer a potential solution to address AMR, recognized as probiotics by the FAO and WHO, these small living organisms, when administered in adequate concentrations, promote intestinal health and host development through fermentation by-products, pH regulation, organic acids (lactic and acetic), EPS, and bacteriocins, which are polypeptide substances17,18.
Currently, probiotics are widely used in the manufacture of fermented dairy products, fruit, meat, sausages, fish, freeze-dried and functional foods. It is also increasing as an alternative for lactose-intolerant people and in vegetarian diets19. However, the application of probiotics is limited since it must face several adverse conditions (environmental, gastrointestinal, and industrial processes) that affect viability and survival20.
Therefore, the microencapsulation of probiotics is used in biotechnology and refers to cover substances or elements for protection against temperature, pH, enzyme activity and controlled release of viable probiotic cells17. The characteristics of the microencapsulation may vary according to the microencapsulating material or matrix and matrices recognized as prebiotics such as maltodextrin and inulin that are generally used20,21.
Maltodextrin is chosen as a microencapsulating agent due to its solubility in water, low viscosity, and clear solution. Additionally, it is easily digested. This allows microencapsulated probiotics to be released and influenced by the gastrointestinal system during digestion. On the other hand, inulin, a fructooligosaccharide with slightly branched structure, is composed of fructose units linked by ß-(2-1) bonds21. Inulin, partially soluble in water, resists human digestion due to its glycosidic bonds. Nevertheless, it supports intestinal microorganism growth. Consequently, inulin acts as a colonic release biopolymer, maintaining its integrity during passage through the upper digestive tract and releasing bioactive compounds in the colon through enzymatic and fermentative processes20,21.
Intestinal cell cultures are valuable tools for assessing the potential of probiotic bacteria and prebiotics in terms of their ability to adhere to intestinal cells, modulate immune function, and promote intestinal health22.
Lactic Acid Bacteria (LAB) microencapsulation involves creating a protective barrier to enhance probiotic activity20. The objective of the study was to evaluate microencapsulated Lactobacillus casei ATCC 393® under in-vitro conditions simulating the gastrointestinal environment and its inhibitory potential on Staphylococcus aureus ATCC BAA 1708®.
Materials and methods
This research was carried out in the PROBIOTEC-FORAPIS Research Group Laboratory located in the teaching laboratory block and in the specialized laboratories of the University of Nariño in the city of Pasto, located in the department of Nariño, Colombia, for year 2022.
The L. casei and S. aureus strains were used for the study. The strains were reconstituted, sown, and inoculated23. The strains were purchased from the distributor MDM cientifica.
Test inhibition L. casei against S.aureus and antibiogram
To evaluate the inhibition of L. casei against S. aureus different methods were used, including impregnated agar discs, method pads with supernatant, diffusion in plastic cylinder with supernatant, and diffusion in double-layer plastic cylinder with supernatant. These methods were conducted under various conditions such as pH 6, filtered (F), unfiltered (UF), heat exposure at a temperature of 80°C for a duration of 10 minutes, both with and without thermal processing24,25. The antibiotic resistance profiles of the L. casei and S. aureus strains were determined with specific antibiotics26. Test performed prior to microencapsulation.
Fermentation Kinetic:
Fermentation kinetics were studied using MRS culture medium and PRO medium for the growth of L. casei. Colony Forming Units (CFU/mL)27, sugars consumed (mg/L)28, protein production (mg/L)29, pH, and lactic acid percentage.
The specific rate of growth was estimated with equation 1, The cell duplication time (dt) was calculated by equation 2, the generation time (g) was calculated using equation 3, the growth rate (K) was calculated using equation 4, units are expressed in generations/hour (K), and the maximum harvest was calculated by equation 5.
Peptide identification, lactic acid analysis, an amino acid profiling
Peptide identification, lactic acid analysis, and amino acid profiling were conducted using HPLC. The peptide profile was determined by HPLC, while lactic acid was measured from the filtered supernatant. Amino acid profiling was carried out for L. casei and S. aureus grown in MRS and BHI broth, respectively. L. casei growth at 37 °C and 45 °C: L. casei growth was analyzed under two different temperatures30.
Microencapsulation by spray drying:
L. casei microencapsulation by spray drying, study, and characterization. A 500 mL solution with a concentration of 10 % w/v was prepared for L. casei. The process utilized a Spray Dryer Bilon 6000s, operating at an input temperature of 170 °C and an output temperature of 67 °C for a 4-hour cycle. The resulting microencapsulated product was sterilized, stored in metallized Ziploc bags, and kept at room temperature (19+2 "C)31,32. To evaluate the microencapsulated material and the encapsulant binary matrix, some aspects were used as stability criteria: viability, efficiency, humidity, water activity, solubility, wettability, morphology, and particle size31,32.
The microencapsulated material was evaluated after 90 days of storage. Viability percentage (equation 6)32, and efficiency33, were determined using specific equations 6 and equation 7.
Humidity was measured using a moisture analyzer, and water activity was determined with Hygrolab Rotronic team (Nürnberg, Germany). Solubility was evaluated by measuring the remaining solids after centrifugation and equation 8. Wettability was determined using the static wetting method32.
Simulated gastrointestinal
Microencapsulated L. casei was also subjected to simulated gastrointestinal conditions to evaluate its behavior34,35. The process involved exposure to lysozyme activity, pepsin, NaCl, HCl, pancreatin, bile, pH (2,5-6,8) and NaOH. Bacterial viability was assessed, and plaque counts were performed27. Exopolysaccharide production was examined at different temperatures and time periods (28±2 °C / 7 days; 35±2 °C / 48 hours and 42±2 °C / 24 hours), the presence of EPS was determined by the appearance of mucoid colonies and confirmed through alcohol mixing36,37.
Intestinal adhesion assays will be conducted to assess the adhesion of L. casei and S. aureus using the mucin from stomach porcine-Type III medium (Sigma-Aldrich)38. The process will involve activation of bacterial strains, harvesting and purification of cells (9000 rpm for 10 min at 4 °C), dilution of biomass with 1 mL of sterile saline solution, and evaluation of cell adhesion (80 μL of prepared sample and anaerobic incubation for 24 hours at 37 °C).
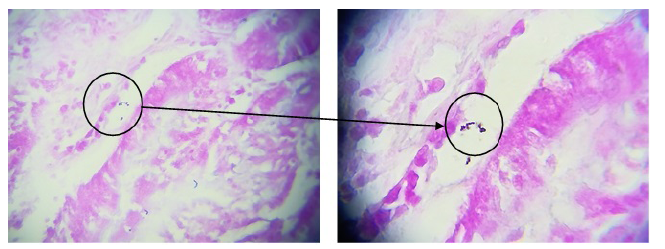
After incubation, non-adherent bacteria will be removed, samples will be stained using the Giemsa stain39 and they will be observed under the microscope to analyze adhesion to mucin.
Statistical design: A descriptive evaluation of the results was carried out to determine their mean and standard error. Each variable had 5 replicates for its determination.
Results
The results obtained for the antibiogram in L. casei indicated resistance against ampicillin (AM 20 μg), florfenicol (FFC 30 μg), and penicillin (P 10 μg), sensitivity against ciprofloxacin (CIP 5 μg), gentamycin (CN 10 μg) and tetracycline (TE 30 μg).
The results obtained for the antibiogram in S. aureus express the strain resistant to cefquinone (CEQ 30 μg), penicillin (PEN 10 μg) and sensitive to amoxicillin (AMC 30 μg), gentamycin (CN 10 μg), doxycycline (D 30 μg), florfenicol (FFC 30 μg), and tetracycline (TE 30 μg). Summary of the inhibiting halos and the respective evaluation methods used under different conditions (See figure 1). The agar disc method was not done with the supernatant but specifically with the lactic strain.
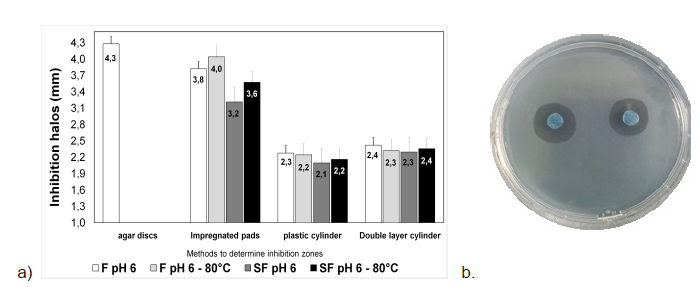
Source: authors.
Figure 1 a) Inhibition halo in mm and methods for determining inhibition under different concentrations of L. casei on S. aureus. b) Inhibition halos of L. casei on S. aureus using the agar disk method. *F filtered. **SF Unfiltered.
The results indicate that the probiotic strain's supernatant possesses antimicrobial activity under the tested conditions and methods. The agar disc method, with L. casei, demonstrated a stronger inhibitory effect against S. aureus.
The data obtained for Ln CFU/mL of L. casei growth in fermentation kinetics indicate differences between culture media (PRO and MRS) (p<0,05) and differences between the times sampled for the PRO medium (9 times) (p<0,05). The MRS medium showed no differences between times (p>9).
For the MRS medium, the exponential phase is obtained at 15 hours with 22.8 Ln CFU/mL (5,6x109 CFU/mL); in the PRO medium, the exponential phase is observed at 18 hours with 28.48 Ln CFU/mL (2,3x10" CFU/mL). The results obtained from fermentation kinetics are described below: specific growth rate of 0,264 (MRS) and 0,698 (PRO); cell duplication time 2,63 hours (MRS) and 0,993 hours (PRO); number of generations per hour 0,381 (MRS) and 1,007 (PRO); maximum harvest 3,73 Ln CFU/mL (MRS) and 9,61 Ln CFU/mL (PRO), exponential protein production 2,43 mg/L (MRS) and 4,86 mg/L (PRO) (See figure 2a); consumption of sugars for the exponential phase 4,96 mg/L (MRS) and 7,68 mg/L (PRO) (See figure 2b); acidity 1,32 % (MRS) and 1,75 % (PRO) (See figure 2c); pH, 4,07 (MRS) and 3,86 (PRO), with a pH range between 5,84 and 3,81 (MRS) and 5,82 to 3,74 (PRO) (See figure 2d).
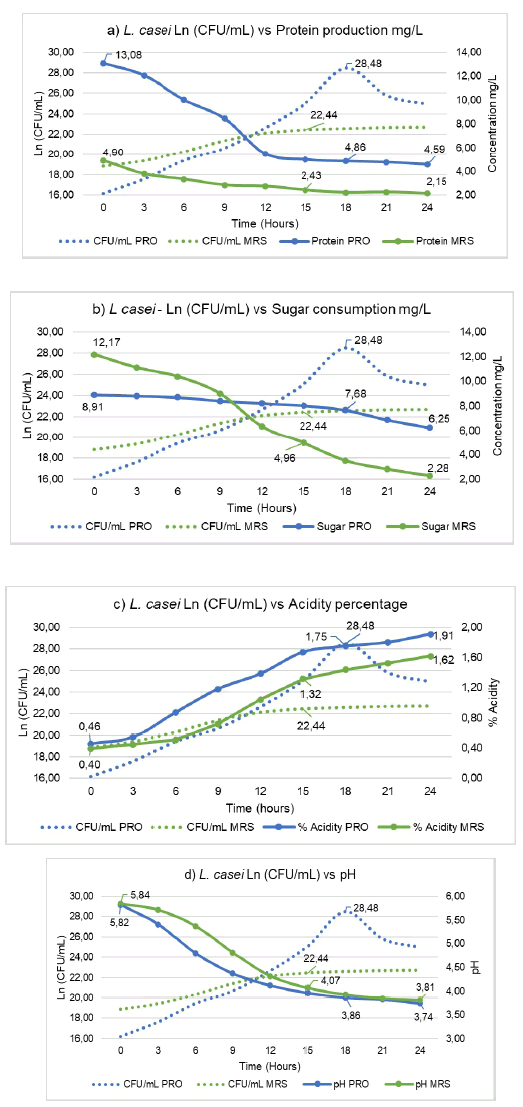
Source: authors.
Figure 2 a) Determination of protein production of L. casei in PRO and MRS medium. b) Determination of sugar consumption by Lactobacillus casei in PRO and MRS media. c) Determination of acidity percentage of Lactobacillus casei in PRO and MRS medium. d) Determination of the pH of Lactobacillus casei in PRO and MRS medium. CFU: Colony Forming Units
Growth of L. casei at 37 °C and 45 °C, statistical analysis indicated differences between 37 °C and 45 °C temperatures (p<0,05). Bacterial growth was recorded for dilutions 10-9, 10-10, 10-11, and 10-12, with values between 1,6x1012 CFU/mL and 3,6x1014 CFU/ mL at 37 °C and values between 1,6x1012 CFU/mL and 1,0x1013 CFU/mL at 45 °C. The optimal growth temperature for L. casei ranges from 35 °C to 40 °C and can tolerate temperatures between 2 °C and 50 °C40,41. The temperature fluctuation in lactic acid production between 29 °C and 42 °C for L. casei is reported as not significant40.
Microencapsulation presented the following results after 90 days of storage: 100 % viability; 84,64 % efficiency; 4,0 % humidity; 99,8 % solubility; 2 min with 22 seconds of wettability; 0,617 water activity; and a particle size ranging between 2,1 pm and 5,28 pm (see figure 3).
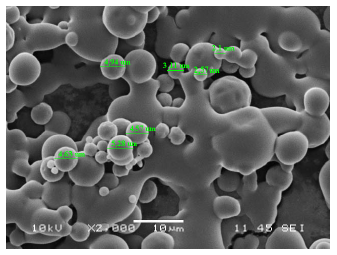
Source: authors.
Figure 3 Scanning electronic microphotography of microencapsulated Lactobacillus casei.
The results after exposing the microencapsulated L. casei against recreated gastrointestinal conditions are presented in Table 1.
Table 1 Bacterial growth of microencapsulated Lactobacillus casei under recreated gastrointestinal environments.
| Continuum conventional | Discontinuous conventional | ||
|---|---|---|---|
| CFU/mL | Lysozyme 10 min | Pepsin+NaCl+ HCl 90 min | Pancreatin+bile+bile salt +NaCl+NaOH 150 min |
| 9,12x1011 | 1,6x1012 CFU/mL | 1,33x1012 CFU/mL | 7,58x1011 CFU/mL |
CFU: Colony Forming Units
Source: authors.
The production of L. casei exopolysaccharides in MRS medium at different temperatures and times was positive and was determined by the presence of precipitate in the evaluated samples.
In the assays conducted to evaluate the adhesion capacity of L. casei, the quality of negative controls (slides without bacterial cells) and positive controls (S. aureus) was initially assessed to verify the purity of the slides and the quality of negative controls. Thus, the adhesion results for L. casei demonstrated its adhesion capacity (see figure 4).
Discussion
Bacteria of the genus Lactobacillus resistant to ciprofloxacin, erythromycin, gentamicin, and vancomycin have been detected in isolates from dairy products such as yogurt, white cheese, tulum cheese, cokelek, camiz cream, and kefir, dairy products that were collected from several supermarkets in Turkey42. Other authors isolated lactic acid strains from neonatal calves (20 to 25 days old) taken from the duodenum, jejunum, and colon, which were reported to be resistant to vancomycin and sulfonamides43. Researchers revealed that some resistant LAB (L. casei, L. plantarum and L. helveticus) to vancomycin and sulfonamide44, ciprofloxacin, tetracycline, and erythromycin carried chromosomal genes (gyrA, tetM, and ermB), which confer resistance to such antibiotics, and these resistance genes are not transferable because they are at chromosome level44,45. The microorganism are not accepted by any regulatory body to be used as a probiotic if it is shown that there is exogenous resistance and that it is easily transmissible43,44, since some strains of lactic acid bacteria could transmit antimicrobial resistance genes to pathogenic bacteria46.
A study in Valledupar (Colombia) in the year 2019, where S. aureus strains were isolated from coastal cheese expressed resistance to tetracycline, chloramphenicol, penicillin and erythromycin47. It is explained that the mecA gene integrated into the chromosome of the S. aureus strain resistant to methicillin is responsible for this characteristic48. This property has been improved and multiplied gradually; therefore, infection of this microorganism in the population has increased49. The antibiograms obtained in the present investigation are like those mentioned above with some exceptions due to the fact that resistance to some antibiotics is determined by the origin of the strains evaluated, both lactic and pathogenic.
The effectiveness of L. casei on the control of S. aureus has been demonstrated by indicating that the inhibition produced by lactic strains responds to various mechanisms of LAB survival50. One of the inhibition mechanisms is the result of organic acids (lactic acid and acetic acid), which is based on the ability of the non-dissociated form of organic acid to cross the cell membrane and cause lysis51.
Furthermore, the identification of the caseicin bacteriocin produced by L. casei, which affects the biosynthesis of proteins and DNA52. L. casei has a significant inhibitory effect on S. aureus, with the agar disc method being the most effective. Antibiotic resistance profiles are essential to ensure the safety of probiotic strains. These results support the potential application of L. casei as a probiotic agent in the prevention of S. aureus infections.
The values for cell duplication reported in different investigations are variable, 14,47 minutes for L. plantarum in MRS medium53, 64,38 minutes and 48,41 minutes for L. gasseri evaluated in MRS and PRO medium54, 0,98 hours and 42,42 minutes for L. platarum in the media MRS and PRO55. In the determination of kinetic parameters of lactic acid bacteria, authors record different values referring to the conditions of microencapsulation, storage period and packaging since they can interfere with each one of the evaluated items.
Around a specific velocity, L. plantarum evaluated in MRS and MSL bacterial media (molasses, whey, and yeast) presented values of 0,61 pmax (h-1) and 0,56 pmax (h-1), respectively56. Moreover, L. casei in culture medium with aloe vera reported 2,7 pmax (h-1) and 2,9 pmax (h-1) in MRS medium57. In a culture medium with inulin, values of 0,79 max (h-1) and 0,307 max (h-1) were recorded for the lactic strains L. acidophilus and L. casei, respectively58, which are data that are similar to those obtained in this study.
The generations per hour and maximum harvest are parameters that, like those mentioned above, vary depending on culture medium, bacterial strain and factors involved in bacterial growth. Thus, 4,62 data points for the number of generations per hour have been found, with a maximum harvest of 10,3 Ln CFU/150 pL59.
Data have been reported for protein production and sugar consumption for several lactic species, as follows: L. gasseri in MRS and PRO culture media of 0,66 mg/L at 20 hours and 3,12 mg/L at 16 hours58, on the other hand, L. plantarum in MRS and PRO culture media of 1,61 and 1,47 mg/L at 16 hours54 for protein production. Furthermore, sugar consumption was recorded in MRS and PRO media in the exponential phase 1,79 mg/L (20 hours) and 2,043 mg/L (16 hours), respectively, for L. gasseri60, and for L. plantarum, a sugar consumption of 6,98 mg/L in the exponential phase (11:50 hours)59.
There are reports of lactic acid percentage in L. platarum evaluated in MRS medium ranging from 0,17 % in zero hours to 0,41 % in 24 hours55. These values are far from those obtained in the study; however, the increase in lactic acid was evidenced. Similarly, it is considered that to obtain good bacterial growth in LAB, the pH has to approach to 5,5 and it is possible that they resist a pH of about 2,061. The bacteriostatic effects of LAB are considered to be related to the production of lactic and acetic acid, as derived from the fermentative metabolism of carbohydrates; in addition, the pH of the medium decreases as an effect of the concentration of organic acids, which correlates with the inhibition of pathogenic microorganisms62.
It should be noted that the study compares the use of various sources of carbon and nitrogen; therefore, the variables obtained from fermentation kinetics will be affected. Various sources of nutrients influence bacterial metabolic processes, affecting CFU population, bacteriocin synthesis, exopolysaccharides, and antimicrobial activity62,63.
L. casei indicated a peptide equivalent to the VAL-TIR-VAL chain at a concentration of 0,56 mg/mL and the detection of lactic acid within the supernatant, characterized by its measured values 27,7 g/L and 29,62 g/L, similar to the value of 30,21 g/L in supernatant of L. casei23. The amount of peptides and amino acids produced by fermentation may be affected by differentiations within the framework of proteolytic metabolism compounds, where nutritional requirements, intracellular peptidases, and their regulatory methods affect the release into the environment64.
Researchers reported values of L. plantarum microencapsulated as follows: 83,3 % viability, 88,4 % efficiency, 7,97 % and 5,23 % humidity, 0,4 % water activity, 1 min with 56 seconds wettability, and 96 % solubility, and microcapsule dimensions of 35,68 pm and 3,47 pm60,65. The results cited are like those reported in the present research and are within stable ranges, except for water activity, which despite having a high value did not affect the results of the other parameters. The interaction between the binary matrix and the microencapsulated bacterium demonstrates stability in the structural composition over a 90-day storage period.
The authors evaluated the microencapsulation of Bifidobacterium BB-12 by spray drying using reconstituted skim milk, inulin and oligofructose as wall material, the authors noted that inulin exerted a protective effect on the bifidobacteria in the encapsulation process and explained this by the possible thermoprotective function that this component exerted on the bacteria subjected to the drying procedure66.
In the literature review, values close to those obtained are found for L. platarum strains with growths between 2,0x109 CFU/150 μL and 3,0x1012CFU/150 μL, and for microencapsulated L. reuteri, a bacterial growth equal to 2,2x1011 under conditions like those assessed in this investigation65. The tests carried out with LAB in the gastrointestinal category depend on the animal species and its physiology. Generally, probiotic strains have to be analyzed under simulated gastrointestinal tract systems in order to determine tolerance to conditions similar to those established by the gastric environment, such as antimicrobial enzymes (lysozymes), low pH, and bile salts67.
In one study, a new synbiotic functional drink powder was designed with extracts of grape pulp, pomegranate and beet peels, encapsulated Lactobacillus casei (quince gum and sodium alginate), the probiotic survival rate in functional drink powders containing free Lactobacillus casei was 42,16 % and increased to 86,40 % and 87,56 % in powders containing microcapsules with sodium alliginate and microcapsules with probiotic sodium alliginate-quince gum, respectively. The production yield (10,95-13,16 %), moisture percentage (4,94-5,17 %), solubility (85,25-88,29 %) and wettability (21,56-22,12s), together with the recommended bacterial survival of 107 CFU/g during the 60-day storage period made the powdered beverage a functional symbiotic product68.
The LAB could produce EPS, whose function is to protect bacteria from factors such as environment and gastrointestinal conditions14. The importance of EPS-producing strains is highlighted in the food industry as polymers that improve the viscosity and texture of products69 and the extraordinary properties of the biopolymers they produce. They do not involve any danger to health, are generally recognized as safe (GRAS), and have antioxidant activity, antibiotic activity, and antitumor activity70.
Various standards have been put forward for the identification of promising probiotics, such as the ability to adhere to intestinal cells. Due to this adherence capacity, the persistence of probiotic strains in the intestine can be enhanced, allowing them to exert beneficial functions such as balancing mucosal immunity, enhancing cytokine production, IgA secretion, inhibitory substance production, phagocytosis, maturation of intestinal epithelial cells, and nutrient absorption53.
It should be noted that the intestinal microbiota consists of consortia of bacteria that exert relevant defense activities and are not attached to the epithelium. Instead, they remain active in the intestinal lumen, aiding in waste elimination and neutralization of toxins and pathogens. In this regard, several investigations have indicated that Lactobacillus strains possess the ability to impede the attachment of pathogens by hindering their establishment through competitive exclusion, a finely tuned mechanism reliant on both probiotic and pathogenic bacterial strains22.
Cellular adhesion is a multifaceted procedure that encompasses the interaction between the probiotic strain and mucus, thereby adding complexity to the interplay of long-distance electrostatic and van der Waals forces, in addition to other interactions at shorter ranges53.
Conclusions
L. casei presented inhibitory action on S. aureus in the methods used. The halo of major inhibition was presented by the method of agar discs with 4.3 mm. The microencapsulated material presented good properties after being stored for a period and subjected to gastrointestinal conditions. These properties are reflected in the different stability tests carried out. The shape of the capsule is circular, with diameters between 2.1 pm and 5.28 pm. The kinetic parameters indicated a logarithmic growth phase in hours 18 (PRO mean) and 15 (MRS mean), with bacterial growths equal to 28.48Ln CFU/mL and 22.44Ln CFU/mL, respectively, and adhesion of L. casei on the mucin from stomach porcine-Type III medium.