INTRODUCTION
Orthodontic treatment aims to establish adequate dental harmonization based on physiological and aesthetic criteria. In the search for these therapeutic objectives, the biological state of the periodontal structures surrounding the tooth may be affected by tooth displacement. Although orthodontic treatment has been indicated to promote gingival health, particularly interdental papillae, by obtaining an adequate position of the teeth and the contacts between them, other studies show serious concerns about the occurrence of pathological events in orthodontic therapy.1,2 One of the most noted unwanted effects is gingival hypertrophy (GH), whose prevalence is more than 50% in studied populations.3
GH is an uncontrolled increase in gingiva volume, which can be spontaneous or induced and may be accompanied by gingival bleeding, periodontal disorders, and even occlusal alterations.4 In addition of GH in patients with orthodontics, other types of gingival hypertrophy have been identified, including GH associated with systemic diseases, neoplastic GH and drug- induced GH.5,6,7 Reports indicate that GH in orthodontic patients starts from the first month after placing the fixed appliances.8 Although its pathogenesis remains unknown, mechanical irritation by orthodontic bands, chemical irritation by the cements used, the impact of food, and poor oral hygiene have been reported to be inducing factors of GH during orthodontic treatment.9,10 Other authors suggest that a low, continuous dose of nickel released to the epithelium is a trigger of this pathology.11,12
Histologically, orthodontic patients with GH have hyperplastic and acanthotic tissue with the presence of altered epithelial cells.13 In addition to thickening and fused epithelial crests, changes in connective tissue related to the accumulation of extracellular matrix (ECM) components have been reported.14 In this altered or fibrotic tissue, the relative amount of collagen is likely to vary. Collagen is the most abundant ECM molecule, which is synthesized by gingival fibroblasts. It acts as a buffer and provides mechanical forces and elasticity to the gingival connective tissue.15 In healthy gingiva, type I collagen (90%) and type III collagen (8%) are the most abundant. The former consists of thick and large fibers, while in the latter the fibers are short, thin and located under the basal membrane and around blood vessels.16
In patients with genetic and drug-based GH, there is a buildup of type I collagen.17 This collagen deposit might be related to the increase in HSP47 (heat shock protein 47) involved in the secretion of type I collagen under the effect of TGF-beta.18 However, there is not enough data on type III collagen and its location in fibrotic tissues, particularly in orthodontic patients with GH. The aim of this study was to determine the presence and distribution of type III collagen in the gingival tissue of orthodontic patients with GH.
METHODS
In this research, 12 patients were selected and divided into two study groups. The first group consisted of periodontally healthy subjects (pink gingiva and no bleeding) with no orthodontic appliances (control; n=6) and the second group consisted of patients with clinical diagnosis of GH and orthodontic treatment (patients; n=6). Participants were seen at the Gingival Hypertrophy Reference Center of the Universidad de Cartagena’s School of Dentistry (Cartagena, Colombia). The inclusion criteria were as follows: patients over the age of 18, in good systemic health, who were not undergoing pharmacological treatment, were diagnosed with generalized GH (≥ 30 in mouth) and had ongoing orthodontic treatment (≥ 1 year), located in the interdental and marginal gingiva without exceeding 1/3 of the clinical crown, and O’Leary plaque index of 15%. Patients with periodontitis, radiographic signs of bone loss, smokers, and individuals undergoing periodontal surgeries less than 1 year prior to the study were excluded. All were informed of the study’s objectives, and they all filled out and signed an informed consent. This research project was approved by the Universidad de Cartagena’s Ethics Research Committee as recorded in minutes No. 94 of 16/02/2017. The procedures carried out complied with the ethical standards set out in the Helsinki Declaration and Resolution 008430 of 1993 of Colombia’s Ministry of Health.19
Sample preparation
Patients were initially subjected to an extra- and intraoral clinical examination by an oral pathologist. In the control group, samples were obtained after coronal elongation surgery for prosthetic or aesthetic reasons. In the GH patient group, biopsies were collected after a gingivectomy. Both interventions were performed by a periodontist under infiltrative anesthesia with 2% Lidocaine and 1:80000 epinephrine in the surgical area. An incision was made to external and intrasulcular bezel with Bard Parker scalpel leaf No. 15. A 7-day post-surgical assessment allowed checking an adequate tissue healing in all participants, without complications. Tissues obtained were deposited into Eppendorf tubes, rinsed immediately in phosphate buffered saline solution (1X PBS, Gibco™) and cut into 3-5 mm3 segments. They were then fixed for 48 hours in 4% buffered paraformaldehyde.
The tissues were then dehydrated in a series of ethanol solutions (30%, 60%, 80%, 95%, 100%) for 5 minutes each, followed by xylene baths (Leica Biosystems) and included in paraffin. For each obtained block, 5 μm serial section were made in a Leica RM2125 RST microtome. The section were mounted on polysine adhesion slides (Thermo Scientific™). Finally, three consecutive slides of the same block were selected for each patient for hematoxylin-eosin (HE) and Masson-Goldner (MG) staining, and type III anti-collagen immunohistochemistry. All procedures were performed at the Research Unit of Basic Dental Sciences of the Universidad de Cartagena.
Hematoxylin-eosin stain
For H&E staining, tissue sections were deposited in xylene baths (Leica Biosystems) and rehydrated in alcohol (100%, 95%, 80%, 60%, 30%). Then they were stained with Mayer hematoxylin (5 minutes) and washed in distilled water. Acid-alcohol differentiation was carried out for 30 seconds, followed by eosin coloration in eosin for 3 minutes and a final rinse in distilled water. Finally, the slides were dehydrated, rinsed in xylene, and mounted on an anhydrous mounting medium (DPX, Sigma-Aldrich). Observations of shape and epithelial and connective tissue cells were made under a Leica DM 500 optical microscope with a built-in camera.
Masson-Goldner stain
After deparaffinizing and rehydrating the slides in alcohol, they were submerged in Mayer hematoxylin for 20 minutes, rinsed in water and incubated for 30 seconds for acid-alcohol differentiation. The slides were then colored in three solutions: azofloxin solution (10 minutes), phosphor plastic- orange acid solution G (1 minute) and green light (2 minutes). After each step through the above solutions, samples were distilled in water. Finally, the slides were mounted with DPX (Sigma-Aldrich).
Immunohistochemistry
The serial sections of gingival tissue of the two study groups were deparaffinized in xylene and rehydrated in ethanol. Antigen recovery was performed by incubating the sections in citrate buffer (pH 6) for 20 minutes at 97°C. Endogenous peroxidases were blocked with 3% hydrogen peroxidase for 15 minutes. The sections were rinsed 3 times for 5 minutes with washing solution (Tris buffered saline (1X TBS, Gibco™ + polyoxyethylene monolaurate), 20 sorbitan or Tween®20 to 0.05%). Specific sites of the sections were then blocked with normal horse serum (Vector Laboratories) for 20 minutes and then they were incubated with anti-collagen type III primary antibody (Mouse monoclonal antibody, dilution 1:500, clone FH-7A, Sigma-Aldrich), overnight at 4°C. The next day, 3 rinses for 5 minutes each were performed in the washing solution. The sections were then incubated for 30 minutes in an anti-mouse biotin secondary antibody (Goat Anti-Mouse IgG Antibody (H+L), Biotinylated, R.T.U. BP- 9200, dilution 1:200 - Vector Laboratories) at room temperature. This was followed by three 5-minute rinses in washing solution and incubated with R.T.U. VECTATASTAIL® ABC (Vector Laboratories) for 30 minutes to visualize the markings. Immunostaining was revealed using a peroxidase substrate solution for 4 minutes. The reaction was stopped in successive rinses with distilled water. Finally, the sections were counter- colored with Mayer hematoxylin for 30 seconds, dehydrated in ethanol, xylene and mounted with DPX. Observations were made on a Leica DM 500 optical microscope connected to a camera.
RESULTS
Of the 12 patients, 50% (6) were female. The average age was 22.8 years in the control group and 20.8 years in the group of orthodontic patients with GH. The control group’s gingival tissue samples stained with hematoxylin-eosin showed a stratified epithelial tissue, with a thin layer of keratin and epithelial cells with defined, round, or oval nuclei (Figure 1A). There were multiple epithelial extensions targeting the underlying connective tissue, a basal stratum with round or flattened nuclei, and a continuous basal membrane delimiting the connective epithelial tissue (Figure 1B). In the connective tissue, there were fusiform-looking gingival fibroblasts with elongated nucleus and surrounded by collagen fibers. These fibers were evenly distributed throughout the tissue, and some of them were seen around the blood vessels (Figures 1A, B, and C).
In orthodontic patients with GH, a keratinized, hyperplastic epithelial tissue with elongated epithelial prolongations was observed, with some of them fusing together (Figure 1D). There was a basal stratum composed of keratinocytes poorly organized and with round nuclei, as well as a basal membrane delimited with dense- looking fibrillary elements (Figure 1E). The underlying connective tissue had multiple gingival fibroblasts surrounded by irregularly arranged and densely distributed collagen fibers. Blood vessels were also seen. There were some inflammatory cells, but these did not have the distinctive appearance of an inflammatory infiltrate (Figures 1D, E and F).
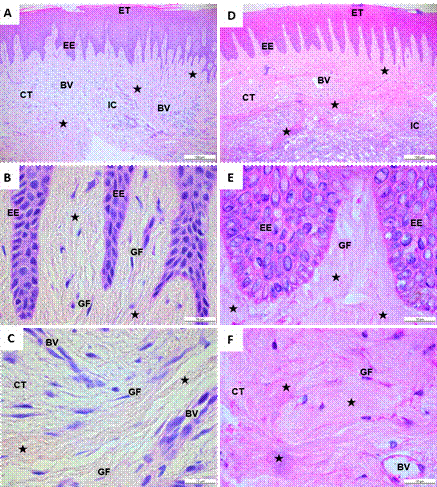
Source: by authors
Figure 1 Histological cuts of gingival tissue stained with hematoxylin-eosin. A, B, C. Gingival tissue of a healthy individual without orthodontic apparatus. D, E, F. Orthodontic patient with gingival hypertrophy. A, D. Organization of epithelial and connective tissue of a healthy individual and a patient with gingival hypertrophy. B, E. Presence of collagen fibers close to EE. C, F. Connective tissue with the presence of collagen fibers, blood vessels, and gingival fibroblasts. Note the abundant presence of collagen fibers in patients with gingival hypertrophy. ET: epithelial tissue; EE: epithelial extensions; CT: connective tissue; GF: gingival fibroblasts; BV: blood vessels; IC: inflammatory cells. Black star: collagen fibers. A, D. Black bar: 100 µm. B, C, E, F. black bar: 10 µm.
The histological assessment of gingival tissue samples colored with Masson-Goldner stain showed the presence of collagen fibers throughout the extent of the connective tissue accompanied by gingival fibroblasts and blood vessels (Figure 2). In the samples of the group of individuals without orthodontic apparatus, there were long, well-organized collagen fibers, many of them grouped into dense packages. Around these fibers, there were gingival fibroblasts and blood vessels (Figures 2A, B, C). In samples of orthodontic patients with GH, there were abundant collagen fibers organized into dense packages, randomly distributed throughout the connective tissue. The green color, which identifies tissue collagen, was more intense in the gingival sections of GH patients compared to those of patients with no orthodontics (Figures 2D, E, F).
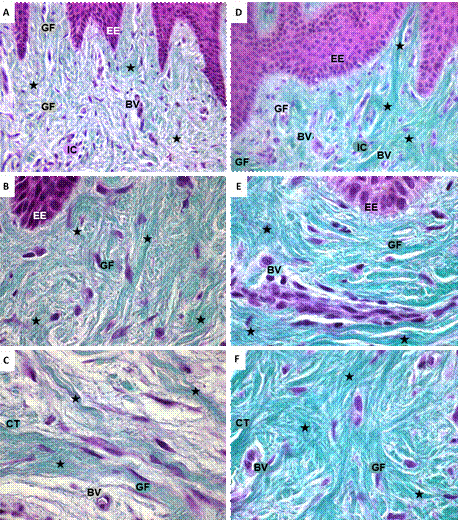
Source: by authors
Figure 2 Microphotograph of histological sections of gingiva colored with Masson-Goldner stain. A, B, C. Distribution of collagen fibers in gingival tissue of individual with no orthodontic treatment. D, E, F. Orthodontic patient with gingival hypertrophy. Green: collagen fibers; nuclei in violet. ET: epithelial tissue; EE: epithelial extensions; CT: connective tissue; GF: gingival fibroblasts; BV: blood vessels; IC: inflammatory cells. A, D. Black star: collagen fibers, 40x. B, C, E, F. Black star: collagen fibers, 100x.
The immunohistochemical expression of type III collagen showed its distribution in gingival tissues of the studied patients (Figure 3). In healthy individuals or those with no orthodontic treatment, type III collagen was predominantly located in the adjacent area of the basal membrane, close to epithelial extensions. It was also observed around blood vessels (Figure 3A, B, C). On the other hand, in gingival tissue samples of orthodontic patients with GH, the distribution and location of type III collagen was also present near the basal lamina, around the blood vessels, but unlike the control group, it was located throughout the extent of the gingival connective tissue. Type III collagen in GH patients was organized into packages that were randomly distributed in the ECM of gingival connective tissue (Figures 3D, E, F). In general, type III collagen immunostaining showed a greater distribution in orthodontic patients with GH compared to gingival tissue samples from healthy individuals.

Source: by authors
Figure 3 Immunohistochemical expression of type III collagen in histological sections of gingival tissue. A, B, C. Immunostaining of type III collagen in gingival tissue of an individual without orthodontic treatment. D, E, F. Orthodontic patient with gingival hypertrophy. A, D. Type III collagen fibers distributed in basal lamina and connective tissue. B, C, E, F. Arrangement of type III collagen fibers in the lamina propia and connective tissue. In brown: type III collagen fibers. EE: epithelial extensions; CT: connective tissue; VS: blood vessels; IC: inflammatory cells. Nuclei in violet. Black star: type III collagen fibers. A, D. Black bar: 100 µm. Black bar: B, C, E, F. 40 µm.
DISCUSSION
GH is characterized by the presence of connective tissue rich in collagen fibers.20 The present study corroborated this connective tissue characteristic, particularly in orthodontic patients with GH. Despite having a reduced number of patients, the samples’ qualitative analysis identified collagen fibers organized into dense packages arranged in various directions of connective tissue in orthodontic patients with GH. These characteristics have already been described by Pêgo et al21 in GH tissues by transmission electron microscopy. However, in samples of orthodontic patients with GH, collagen density, particularly type III collagen, was especially visible. These findings are similar to those reported by Romanos et al,22 who used immunofluorescence techniques and found abundant presence of type III collagen in the gingival tissues of GH patients.
The histopathological analysis in orthodontic patients with GH showed a keratinized epithelial tissue and multiple fused epithelial extensions that penetrate the underlying connective tissue. These results agree with those of Pascu et al,23 who evaluated gingival tissues of three patients who developed GH by using orthodontic appliances, finding out a predominantly parakeratinized epithelium with areas of acanthosis, and large epithelial extensions penetrating the connective gingival tissue. The connective tissue of GH patients evaluated in this study suggests the presence of gingival fibroblasts of fusiform aspects, blood vessels and inflammatory cells in various areas of connective tissue. These findings are similar to those reported by Surlin et al24 and Jadhav et al,25 who also reported a diffuse mixture of dense collagen beams interspersed with variable amounts of fundamental substance.
The reason for a likely increase or accumulation of collagen fibers in the gingival connective tissue of GH patients is unclear and controversial. Kantarci et al26 found increased mitotic activity of gingival fibroblasts, accompanied by decreased apoptosis, while Meng et al27 found a normal or even decreased rate of mitotic activity of gingival fibroblasts. The loss of ECM homeostasis in orthodontic patients with GH shown as a higher deposit of type III collagen is perhaps the result of the imbalance between collagen secretion by gingival fibroblasts and the decrease in degradation activity of the extracellular matrix exerted by matrix metalloproteinases (MMP-1, MMP-2, MMP-3).18 Various studies have linked the increase in the function and production of fibroblasts with different specific cytosines and receptors. In fact, high levels of transforming growth factor beta 1 (TGF-β1) have been reported in patients with GH, which induces increased levels of connective tissue growth factor (CTGF) and this in turn stimulates collagen production by gingival fibroblasts.28
According to the bibliographic review carried out by the authors of the present study, this is apparently the first study that uses the immunohistochemistry technique to show a probable increase in the relative amount of type III collagen in gingival tissue samples of orthodontic patients with GH. However, these preliminary findings should be confirmed in future studies with a greater number of patients. Similarly, the probable increase of interstitial collagen in orthodontic patients with GH should be confirmed by measuring the gene expression levels of different types of collagen, including type III collagen, from gingival fibroblasts in cell culture studies. It would also be helpful to use immunofluorescence technique and polarized light microscopy to identify the presence of different types of collagen in gingival tissue. This could help improve the understanding of the pathological aspects involved in GH in orthodontic patients.
CONCLUSION
Type III collagen appears to be increased and have a wide distribution throughout the gingival connective tissue of orthodontic patients with GH compared to gingival tissues of individuals with no orthodontic treatment. Future studies in cellular models will be useful for clarifying the pathological aspects of gingival hypertrophy.














