Introduction
Substances with one or more unpaired electrons in the outer orbital are known as free radicals (reactive oxygen and nitrogen species) 1. The presence of unpaired electrons makes them reactive, looking for a partner by attacking and binding electrons to molecules around it, including cellular components such as lipids, lipoproteins, proteins, carbohydrates, ribonucleic acid (RNA), and deoxyribonucleic acid (DNA) 2. A further consequence of free radical reactivity is damage to cell structure and function. This state can lead to oxidative stress, conducting to illnesses including degenerative diseases, cardiovascular problems, aging, and cancer 3.
Antioxidants can quench free radicals. The human body produces antioxidant enzymes, such as superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GPx) 1. However, under oxidative stress conditions, the number of free radicals is greater than the antioxidants produced by the body 4. Therefore, antioxidants should be consumed, whether they are of natural or synthetic origin 2. Although, when synthetic antioxidants are used, the potential for toxicity and adverse consequences must be considered 4. Natural antioxidants such as phenolics and their derivatives can be easily found in natural sources, especially plants 2 or potential species such as those from the sea 5.
Exploration of the sea and marine biodiversity provides a new sector in the search for novel natural products with health advantages for humans 6. The chlorophyll seaweed Caulerpa racemosa (Forsskal) J. Agardh is abundant on the coast of Indonesia, where it is traditionally used as fresh vegetables (lalapan) and consumed by residents and fishermen 7. C. racemosa contains phenolic compounds 8, caulerpenyne, lipids, proteins, and minerals, making seaweed a potential functional food 7. The biological activity of C. racemosa has been widely documented, including larvicidal, antibacterial 9, and antioxidant 8 properties.
Extraction is the initial stage in studying the phytochemicals of natural materials 10. The choice of extraction process must ensure the successful extraction of the target molecule 11 and maintain the quality of the resulting extract 12. Different extraction methods can be used to obtain antioxidant compounds, both conventional and unconventional. Conventional extraction methods (including maceration, percolation, Soxhlet extraction, and reflux) are often employed to extract phytochemicals from natural origins, including marine 13. However, unconventional extractions such as supercritical carbon dioxide, microwave-assisted, and ultrasonic extraction have been widely developed and used to extract natural compounds from seaweed 14. Information on the best extraction method to obtain maximum phenolic content in C. racemosa is still limited. Thus, in this study, the extraction procedure of C. racemosa was carried out using several extraction methods, along with testing the antioxidant activity of the extract.
Materials and methods
Powder preparation of C. racemose
C. racemosa collected from around the Taman Mangrove Beach (5(58’30.5” S 106(42’14.2” E), the northern region of Untung Jawa Island, Kepulauan Seribu, Indonesia, in February 2020. C. racemosa was determined at the Research Center for Oceanography, Indonesian Institute of Sciences, Jakarta, Indonesia (B-599/IPK.2/IF.07/VII/2020). Fresh C. racemosa (12 Kg) was cleaned using running water. The material was dried in direct sunlight with a black cloth. After drying, the material was powdered and sieved 15.
Extraction Procedure
1. Maceration
The dried powder (150 g) was put in a glass bottle. A 70% ethanol solvent was used to macerate and extract the powder components at a 1:10 powder-solvent ratio (1.5 L). The mixture was stirred occasionally for the first six h and then left to stand. After 24 h, the filtrate was collected, and the residue was re-macerated with a new solvent for five repetitions 15.
2. Ultrasonic
The dried powder (150 g) was put into a beaker glass and extracted with 70% ethanol solvent with the same sample-solvent ratio as in the maceration method. The extraction process was carried out for 60 min at 30 °C using an ultrasonic bath (Branson 5510, Marshall Scientific LLC., USA) with a frequency of 40 kHz. The filtrate was collected, and the residue was re-extracted using a new solvent for five repetitions 16.
3. Soxhlet extraction
The dried powder (150 g) was extracted with 250 mL of 70% ethanol as a solvent in a Soxhlet apparatus. The extraction process was carried out at 70 °C. Extraction was stopped after the color of the solvent on the siphon became clear. The filtrate was collected. The extraction was conducted with five repetitions 15.
The filtrate obtained from each extraction method was then concentrated using a vacuum rotary evaporator (Eyela, Shanghai, China) at 50 °C, followed by a water bath at the same temperature until a concentrated dry extract was obtained and weighed. The yield extraction percentage was calculated by dividing the weight of the extract obtained (g) against the dried powder of C. racemosa (g) multiplied by 100%.
Determination of extract characteristics
We determined the extracts’ characteristics, such as organoleptic, moisture content, and total ash. The organoleptic examination includes color, odor, taste, and texture. Organoleptic observations were made after the extract was exposed to air for 15 min using the senses (eyes, nose, and tongue) 17. Determination of water content was carried out by the gravimetric method using an oven at 105 (C. The total ash content was conducted by burning 2 g of the extract in a kiln at 600 (C for five h until it became white ash. These evaluations conform to the procedures of Indonesian Herbal Pharmacopoeia (2017) 15 and the World Health Organization (2011) 12.
Phytochemical Screening
Identification of the chemical content (viz. phenolics, flavonoids, saponins, triterpenoids/steroids) in the extracts was carried out following the procedure on Harborne (1987) 18. Identification of phenolics was carried out using 5% (w/v) FeCl3 reagent, flavonoids using magnesium powder and concentrated hydrochloric acid, saponins using the foam test in water, and triterpenoids/steroids using Salkowski (chloroform and concentrated sulfuric acid) and Liebermann Burchard reagents (chloroform, concentrated sulfuric acid, and anhydrous acetic acid). Identification of alkaloids was carried out with Dragendorff, Mayer, and Hager reagents according to procedures on Departemen Kesehatan RI (2000) 19 and tannins using 1% (w/v) gelatin adjusted to the procedure on Hanani (2015) 20.
Determination of phenolic levels
Determining phenolic levels was carried out using gallic acid as a standard. This procedure was initiated by preparing gallic acid stock solution (Sigma Aldrich, Saint Louis, USA) (500 (g/mL) in ethanol and an extract stock solution (1000 (g/mL) in ethanol. Serial concentrations of gallic acid were prepared at 11, 19, 27, 35, and 43 (g/mL in ethanol to determine the calibration curve. Meanwhile, the extract test solution was prepared at 100 (g/mL. This test solution was diluted from the extract stock solution. Test procedure brief description: the test solution of both gallic acid and extract (0.5 ml) was separately added to 0.5 mL Folin-ciocalteu reagent (Merck KGaA, Darmstadt, Germany) (1:10, v/v in water). The mixture was homogenized using a vortex and allowed to stand for 3 minutes. Then, 1.5 mL of 7.5% Na2CO3 was added to the mixture, homogenized, and incubated for 30 min. Using a UV-Visible spectrophotometer (UV-1601 Series, Shimadzu), the absorbance of the mixture was measured at 741 nm. Milligrams of gallic acid equivalent (GAE) per gram of extract (or mg GAE/g) represented the overall phenolic content of the extract 21.
Determination of Antioxidant Activity
Antioxidant activity was determined by the 2,2-diphenyl-1-picrylhydrazyl (DPPH) method 22. The reference Quercetin solution was prepared in 100 (g/mL concentration in methanol (Sigma Aldrich, Saint Louis, USA). This solution was diluted in methanol to 2, 4, 6, 8, and 10 (g/mL. The stock solution of extract was prepared in a concentration of 1,000 (g/mL in methanol and diluted to 100, 200, 300, 400, and 500 (g/mL in methanol. A 200 µL of quercetin or extract solution were mixed with 1 mL DPPH (Sigma Aldrich, Saint Louis, USA) 0.639 mM and 4 mL methanol. The mixture was homogenized and incubated at room temperature for 30 minutes in the dark. Absorption was measured at 515.5 nm using a UV-Vis spectrophotometer (UV-1601 Series, Shimadzu, Kyoto, Japan). The percentage of DPPH radical scavenging (%) is calculated by: (absorbance of the DPPH ( absorbance of the sample)/absorbance of DPPH ( 100%. Then, the percentage of DPPH scavenging (y) is plotted against the sample concentration (x) to obtain a linear line equation y = bx±a. The value of 50 is entered as “y” so that the value of x can be obtained, which is interpreted as a concentration capable of scavenging 50% DPPH radical (IC50). The antioxidant activity index (AAI) was calculated as follows: the ratio of the DPPH final concentration ((g/mL) to the IC50 value of the sample ((g/mL). The criteria for the antioxidant activity of the extracts were measured based on the AAI values, namely: poor (AAI < 0.5), moderate (AAI between 0.5 and 1.0), strong (AAI between 1.0 and 2.0), and very strong (AAI > 2.0) 23.
Statistical analysis
Data on the total phenol content of extracts were tested for homogeneity and normality by the Kolmogorov-Smirnov test. The differences in total phenolic content of the three types of extracts were tested by one-way ANOVA with (=0.05. Meanwhile, the antioxidant effect of the samples was compared based on their IC50 and AAI values descriptively.
Results
We obtained 1.5 Kg dry C. racemosa from 12 Kg of fresh sample. The yield percentages obtained from the 150 g of C. racemosa dried powder by maceration, ultrasonic, and Soxhlet extraction methods were 13.96 %, 16.03 %, and 17.85%, respectively. The results of the organoleptic test of the extracts showed that the three extracts were odorless, slightly salty, and viscose. The extract obtained from the Soxhlet extraction was dark green, while the other extracts were greenish-brown. Table 1 lists the water content and total ash in each extract.
Table 1 Water and total ash content of C. racemosa ethanol extracts
| Extraction methods | Water content (%) | Total ash content (%) |
|---|---|---|
| Maceration | 7,96 ± 0.84 | 20.55 ± 0.63 |
| Soxhlet | 7.17 ± 0.04 | 25.71 ± 0.33 |
| Ultrasonic | 7.65 ± 0.01 | 22.74 ± 0.63 |
Evaluations in triplicate.
The phytochemical screening results showed that all extracts contain phenolics, flavonoids, tannins and saponins (Table 2).
Table 2 Phytochemical of C. racemosa ethanol extracts
| Extraction methods | Phytochemical | |||||
|---|---|---|---|---|---|---|
| Phenolics | Flavonoids | Tannins | Alkaloids | Saponins | Steroid/tri terpenoids | |
| Maceration | + | + | + | - | + | - |
| Soxhlet | + | + | + | - | + | - |
| Ultrasonic | + | + | + | - | + | - |
Evaluations in triplicate; (+) = detected; (-) = not detected
The total phenolic content of extracts was determined using the calibration curve of gallic acid (y = 0.0111x + 0.1466; R2 = 0.9994.) (Table 3). Based on the one-way ANOVA test, there was a significant difference in the total phenolic content of the three extracts obtained by maceration, Soxhlet, and ultrasonic methods (( = 0.05). The highest total phenolic content was found in the extract produced from the ultrasonic-assisted extraction method of 39.38 ± 0.36 mgGAE/g (ultrasonic > Soxhlet > maceration).
Table 3 Total phenolics content of C. racemosa ethanol extracts
| Extraction methods | Total phenolics content (mgGAE/g) |
|---|---|
| Maceration | 22.05 ± 0.05* |
| Soxhlet | 37.31 ± 0.41* |
| Ultrasonic | 39.38 ± 0.36* |
Evaluations in triplicate. *Means a significant difference in the average total phenolic content based on the three groups of extraction methods ((=0.05).
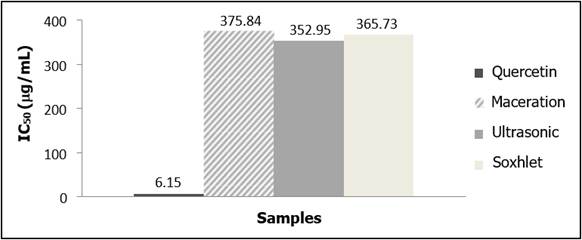
The DPPH technique was used to assess the antioxidant activity of the C. racemosa ethanolic extracts. The IC50 value of C. racemosa ethanol extract produced from the three extraction methods (>300 (g/mL) showed weak antioxidant activity against DPPH radicals when compared to quercetin (6.15 ± 0.07 (g/mL). In this study, AAI values of the C. racemosa ethanolic extracts obtained by maceration, Soxhlet, and ultrasonic were 0.67, 0.69, and 0.71, respectively. Meanwhile, the AAI value of quercetin was 41.04. An AAI value of around 0.5-1.0 indicates a moderate antioxidant of the 70% ethanol extract of C. racemosa23.
Discussion
Marine is a source of natural materials with important chemical content with potential use in medicine, nutraceuticals, cosmetical, and food. Marine metabolites can be extracted and isolated by various techniques 13. This study evaluated the characteristics, total phenolic content, and antioxidant activity of the ethanol extract of C. racemosa using three different extraction methods: maceration, Soxhlet, and ultrasonic.
One of the characteristics of the extract that is determined is the water content. Based on the results obtained, the water content of the three C. racemosa extracts was at <10%. It meant that the extracts still met the quality standard of extract according to Farmakope Herbal Indonesia (Indonesia Herba Pharmacopeia) 17. High water content in the extract can cause contamination of microorganisms, which can affect the quality of the extract 12. Caulerpa spp. naturally grows on the coast. Fresh Caulerpa spp. predominantly contains water, up to 94.84% 24. According to Tapotubun (2018) , drying fresh C. lentillifera under indirect sunlight, covering them with a black cloth can drastically reduce this water content. However, the dried marine algae are still high in water content at 9.22 to 18.22% 24.
Seaweed, including C. racemosa, has a very high mineral content 7. The total ash content of the three extracts was >20%. The ash content in other Caulerpaceae species (C. veravelensis, C. Scalpelliformis, and C. racemosa) was 24.20-33.70% 25; meanwhile, in C. lentillifera was around 40.66-41.83% 24. Total ash indicates the presence of material remaining after ignition. This material can come from the internal or external (environment) 26. A high total ash content indicates the risk of heavy metal contamination such as Hg, Pb, Cd, etc. However, it is better to carry out further qualitative and quantitative identification to ascertain the type and amount of heavy metal in the extract.
C. racemosa contains important phytochemicals, such as phenolic, flavonoids, tannins, saponins, coumarins, carbohydrates 27, fatty acids, and methyl esters 16. The choice of solvent is crucial for selectively extracting the target compound 11. In addition, safety and toxicity considerations must also be considered 28. Ethanol 70% is a mixture of water-ethanol solvent that is known to be able to extract phenolic compounds. This solvent can increase phenolic extraction compared to the solvents used individually 29. Other chemicals, such as tannins, polyacetylenes, flavonols, terpenoids, sterols, and alkaloids, may be extracted using ethanol 30.
The percentage yield of the extract obtained from Soxhlet is the highest compared to other extraction methods (Soxhlet > Ultrasonic > Maceration). However, the highest phenolic content of the C. racemosa extract was found in the extract produced by the ultrasonic-assisted extraction method compared to the Soxhlet and maceration methods (Table 3). Based on the statistical analysis results with the one-way ANOVA test, there was a significant difference in the total phenolic content of the three extracts ((=0.05). Traditional extraction techniques (such as Soxhlet or reflux) typically use a sizable number of solvents, a prolonged extraction period, and high temperatures 29. Although the extract produced by Soxhlet has a higher yield percentage than ultrasonic, the Soxhlet method is suspected to be able to extract compounds other than phenolic, such as chlorophyll pigments 31,32. In addition, there is the potential for oxidation and hydrolysis of phenolic compounds caused by overheating conditions during extraction with Soxhlet 29. Meanwhile, ultrasonic is considered an alternative extraction method that is effective and efficient in extracting marine-derived compounds 13. Ultrasonic waves can cause cell wall rupture, increasing solvent penetration in cells. The use of ultrasonic in phytochemical extraction is based on the physicochemical concept of acoustic cavitation, a phenomenon where ultrasonic waves cause bubbles that grow and burst in a liquid media 16. The ultrasonic-assisted extraction of components from natural materials became the more effective and efficient method in our research. However, the optimal extraction conditions of ultrasonic extraction vary for each species and must be evaluated separately.
The DPPH (2,2-diphenyl-1-picrylhydrazyl) is highly reactive and captures electrons from other compounds to become stable 1. The DPPH method describes the mechanism of action of antioxidants with Single Electron Transfer (SET) 2, and it is used to determine the antioxidants' scavenging ability. This method is valid, simple, accurate, sensitive, and economical. Both pure chemicals and complex samples may have their antioxidant capacity measured 33. In addition, it is quite reproducible compared to 2,2'-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid (ABTS), even though the reactivity of DPPH with radicals (alkyl) 34. The inability to attain a steady state with varying antioxidant/DPPH ratios due to the nonlinearity of the time response curve limits its use 1. Based on this study, the antioxidant in the 70% ethanol extract sample was moderate compared to quercetin. Determination of antioxidant activity with other mechanisms of action can still be determined on extracts that provide the highest phenolic content.
Conclusions
Our findings showed that the extraction process influenced the phenolic content obtained from C. racemosa. A non-conventional method, such as ultrasonic extraction, has many advantages in terms of the efficiency and effectiveness of phenolic recovery. However, C. racemosa extract showed weak antioxidant activity compared to quercetin. The use of C. racemosa as a source of medicinal, nutraceutical, and marine food ingredients still needs further study.