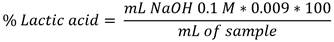Introduction
The functioning of the human body depends on a complex interaction with the native microbiota 1, which are microorganisms that establish a symbiotic relationship with the host and helps maintain physiological homeostasis 2. Native microbiota is different for each individual, and its composition depends on lifestyle, early exposure to microorganisms in their environment, therapy against infections (antibiotics), and the genotype of the individual 1. The individual's diet 3) is a determining factor too.
Nevertheless, the current accelerated lifestyle, the unhealthy eating habits 4, together with factors such as a sedentary lifestyle and stress, are influencing this microbiota 5, stimulating the increase in diseases such as diabetes, obesity, high blood pressure, and cancer 4. For example, a diet predominating animal proteins and fats would be associated with a higher incidence of obesity 3, diabetes mellitus 6, metabolic syndrome, inflammatory bowel disease, and coronary heart disease. Many diseases are even the result of the loss of harmony between our genome and the microbiome 1. On the other hand, a diet with a predominance of plant-based foods, with a good proportion of prebiotics, would seek an intestinal microbiota that is not associated with the mentioned conditions and that could even reverse them. 3
In this sense, consuming "plant-based foods" can become a solution, as they can promote healthy intestinal microbiota. Furthermore, they have been promoted 7 since it was discovered that they provide abundant functional phytochemicals, such as carotenoids, polyphenols, flavonoids, and saponins, which can reduce the risk of chronic diseases 8. For these reasons, plant-based non-alcoholic beverages have achieved a growth rate of up to 15% in the global market in recent years 8.
For preparing these beverages, cereals are proposed as optimal 9, considering they are an important source of nutrients and functional phytochemicals 8. On this basis, quinoa (Chenopodium quinoa Willd.) is one of the most important. It is a dicotyledonous plant belonging to the Chenopodiaceae family 10, considered a pseudocereal native to the Andean region. It contains minerals, proteins, and important phytochemicals such as phenols, isoflavones, saponins, and phytosterols 11. Without having an exceptional amount of protein (from 12 to 23%), it is characterized by its high combination of essential amino acids because it has an ideal balance.
Regrettably, consumers do not entirely accept cereals due to their sensory qualities and because some of their nutrients’ digestibility is inadequate for specific individuals 11. Therefore, they are usually supplemented with enzymatic treatments and microbial fermentations to improve their flavor and nutrient transfer 11. In recent years, interest has focused on obtaining fermented beverages based on cereals or pseudocereals, which can be used as substitutes for fermented beverages 12.
From this perspective, it is possible to formulate products with probiotics 13 and food substances needed to grow lactic acid bacteria 14. Probiotics constitute one of the main functional components and represent an accessible option to the population 15. Lactic acid fermentation produces them with functional ingredients such as short-chain fatty acids, vitamins, and exopolysaccharides 8. Likewise, they play a vital role in maintaining intestinal microecology and preventing chronic diseases 8. Lactic acid fermentation products can even change the taste of food or beverages 11.
For the reasons mentioned above, this research aims to develop a probiotic quinoa beverage that combines plant-based foods with lactic acid fermentation. Therefore, sensory qualities can be improved, and the intestinal microbiota can be positively impacted. Furthermore, the novelty lies in using an enzymatic pretreatment to hydrolyze the starch, considering that few reports use enzymatic hydrolysis 11, and, on the other hand, fermentation can enhance antioxidant activity 16.
Materials and methods
Starch hydrolysis of quinoa flour
Quinoa flour was purchased from local markets in Lima, Perú, in the “Nutrimix” brand. It consisted of crushed and ground white quinoa grains. Its composition contains easily digestible proteins, calcium, iron, phosphorus, and lysine 17. Following the procedure described by 18, a suspension of white quinoa flour ("Nutrimix" brand) was made at 12.5 % w/w. Subsequently, the pH was conditioned to 7.8, and the enzyme α-amylase from Bacillus licheniformis (Sigma Aldrich®) was added at a concentration of 0.01% p/p starch, considering a concentration of 70% starch. Next, it was heated at 100°C for 40 minutes in a water bath (Memmert) with constant stirring. Later, the temperature was reduced to 90 °C, and the hydrolysis was continued for another 60 minutes with continuous stirring. Finally, hydrolysis was terminated by lowering the temperature to 25°C. This stage released the sugars in the quinoa starch to be lactically fermented in the next step. To confirm the occurrence of hydrolysis, soluble solids (°Brix) were measured before and after hydrolysis.
Lactic acid fermentation
A "Vivolac" brand probiotic culture suspension was made at 0.278% w/w. The culture included the genera: Lactobacillus delbrueckii subsp. Bulgaricus (10%), Streptococcus salivarius subsp. Thermophiles (70%), Lactobacillus acidophilus (10%) and Bifidobacterium ssp. (10%) 19. This suspension was added to the hydrolyzed starch solution in three different concentrations: 10 , 5 , 1 , and 0 %, and the suspension was carried to lactic acid fermentation at 42 ± 1°C, under microaerophilic conditions in an incubator (Memmert, Model 854 Schwbach). Three different fermentation times were tested: 8, 10, and 12 hours, obtaining 12 treatments performed in triplicate. These times were determined based on previous tests that stated that pH begins to decrease after 6 hours. Thus, considering that these cultures are for milk 19 and not for quinoa, it was necessary to consider the adaptation time for the microorganisms 20. Finally, the fermentations were stopped by reducing the temperature to 4°C.
For all treatments, four parameters were measured: pH (HANNA portable pH meter, Model HI 8424), total titrable acidity expressed as a percentage of lactic acid (% w/w) (according to the procedure described by 21; reducing sugars by the DNS method, described by Miller, and modified by 22; and soluble solids (°Brix) (with an RHB-55 refractometer). These parameters were used as indicators of the occurrence of fermentation and were measured both before and after fermentation had started.
For total acidity, the following formula was used:
Likewise, a calibration curve was prepared to determine total sugars, and the spectrophotometer readings at 540 nm were transformed into reducing sugar concentration (mg/mL) using the equation y = 6.579 X - 0.2414.
Once all the fermentations were finished, the best treatment was chosen based on its lactic acid production, according to the recommendations of 23. Other parameters: pH, reducing sugars, and soluble solids, were also measured. Still, they were only considered as a reference).
Formulation
The beverage was formulated by adding natural honey (4.375 % w/v), carob (2.5 % w/v), and potassium sorbate (0.01 % w/v). Additionally, the beverage was formulated by adding mango flavor (with cloudy mango flavoring (7 drops/400 mL) from Fratello Importaciones Goicochea EIRL).
Proximal analysis
The beverage was subjected to a proximal analysis in "La Molina Calidad Total" laboratories. The following parameters were analyzed: Total calories (by MS-IN calculation, Collazos 1993); Carbohydrates (by MS-INN difference, Collazos 1993); Fat (AOAC 986.35 (B). Cap 50, Ed.18, Page 18, Revision 4. 2011-2005); Humidity (AOAC 950.27 (B). Chap 29, Ed.18, Page 6, Revision 4. 2011-2005); Ashes (AOAC 950.27 (B). Cap 29, Ed.18, Page 2, Revision 4. 2011-2005); Protein (AOAC 986.13 Chap 32, Ed.18, Page 14, Revision 4. 2011-2005); Crude fiber (NTP.205.003, Revised 2011. 1980); %Kcal from fat (By MS-INN calculation. Collazos 1993); % Kcal from protein (By MS-INN calculation. Collazos 1993); % Kcal from carbohydrates (By MS-INN calculation. Collazos 1993).
Microbiological analysis
The beverage was also microbiologically analyzed two times: immediately after its preparation and two weeks after it. During these two weeks, the product was stored under aseptic conditions at a temperature of 4°C. The parameters analyzed, as requested by the General Directorate of Environmental Health of the Republic of Peru 24 were: Total mesophilic aerobic count by analysis in Plate Count Agar; Count of fungi and yeasts by Analysis in Sabouraud Agar; Total coliform count by analysis in VRB Agar; Lactobacillus count by analysis in MRS Agar. In addition, a simple gram staining of the colonies that grew on MRS agar was also performed.
Statistical analysis
To determine the treatment with the highest production of lactic acid, a 4x3 factorial experiment was used, with three repetitions, suitable for a DCA. Then, the analysis of variance (ANOVA) conducted in DCA was performed, and a mean comparison test (Tukey's test) was applied for the main and simple effects.
Results
Enzymatic hydrolysis
After enzymatic hydrolysis, the measurement of soluble solids (°Brix) indicated that, on average, 7.5 g of soluble solids were released per 100 g of beverage, resulting in between 9 and 10 °Brix at the end of hydrolysis. These results are similar to those reported by 18, who, after following a similar procedure, obtained a final 9 °Brix. Due to the α-amylase from Bacillus licheniformis (Sigma-Aldrich®), these released soluble solids would consist of maltose, maltotriose, maltotetraoses, maltooligosaccharides, and glucose 25, which are susceptible to being fermented by probiotic microorganisms in the next stage. It is worth mentioning that previous tests showed that not adding enzymes led to starch gelatinization.
Lactic acid fermentation
pH
For all treatments, after the fermentation, it was observed a decrease in pH (Table 1), including the control groups, although, in these, the variation was minimal. The greatest pH variations were observed in the 10-hour treatment for 10 % and 5 % inoculum. A decrease in pH below 4.0 would be an indicator of acid production. According to 26, determining pH is essential in monitoring acid production by lactic acid bacteria.
Table 1 pH (± 0.01) at different fermentation times and different inoculum concentrations
| pH (± 0.01) | ||||||
| 8 HOURS | 10 HOURS | 12 HOURS | ||||
| INOCULUM | INITIAL | FINAL | INITIAL | FINAL | INITIAL | FINAL |
| 0% | 6.68a ± 0.02 | 6.57a ± 0.04 | 6.85e ± 0.05 | 6.14e ± 0.91 | 6.80j ± 0.06 | 6.29j ± 0.79 |
| 1% | 6.66a ± 0.02 | 4.29b ± 0.01 | 6.83e ± 0.03 | 4.18f ± 0.04 | 6.74j ± 0.00 | 3.96k± 0.05 |
| 5% | 6.65a ± 0.01 | 4.14c ± 0.04 | 6.81e ± 0.05 | 3.86e ± 0.10 | 6.74j ± 0.03 | 3.90m ± 0.07 |
| 10% | 6.57a ± 0.02 | 4.02d ± 0.03 | 6.79e ± 0.05 | 3.84h ± 0.09 | 6.64j ± 0.04 | 3.88n ± 0.05 |
The same letter indicates that there were no significant differences between initial and final stages.
A T-test was applied to test the differences between the Initial) and the Final pH of the fermentation at 8, 10, and 12 hours. Results indicated significant differences for all treatments (α=0.01), except for those with 0% of inoculum. The medium was rapidly acidified with higher concentrations of inoculum since a higher initial population would allow for reaching the maximum population size in a considerably shorter time 27. However, as the fermentation time increases, the pH values tend to become similar, which could be because the stationary phase has been reached.
Total Titrable acidity expressed as a percentage of lactic acid (%)
As shown in Figure 1, the highest acidity productions occurred in the 10-hour treatment and 10 % inoculum, and the 12-hour treatment for 5 % and 1 % inoculum. These results are similar to those found for the measurement of pH. Likewise, as the fermentation time increases (12 hours), the acidity values tend to become similar, indicating that the stationary phase is being reached. It was also observed that the production of lactic acid at 12 hours with 10 % inoculum is reduced, compared to the concentration with 5 %. According to 28, the accumulation of lactic acid results in adverse effects, such as intracellular acidification, accumulation of anions, membrane disturbances, and increase in turgor pressure, among others.

Figure 1 Total titrable acidity produced at different fermentation times and different inoculum concentrations. Error bars represent standard deviation (3 replicates).
The pH and total titrable acidity results indicate that the microorganisms have adapted and are using the carbohydrates in the quinoa starch to initiate fermentation 23. The acid produced would be mainly lactic acid since the probiotic strains used are mostly homofermentative, and they have almost exclusively lactate through the Embden-Meyerhof-Parnas pathway 26.
Reducing sugars:
Table 2 shows the data on the reducing sugars concentration for all treatments before and after fermentation. In the treatments at 8 hours, for 1 %, 5 %, and 10 % inoculum, an increase in the concentration of reducing sugars is observed. However, in the treatments at 10 and 12 hours, a slight reduction is observed for 1 %, 5 %, and 10 % inoculum.
Table 2 Reducing sugars (± 0.01) at different fermentation times and different inoculum concentrations
| Reducing sugars (mg/mL) | |||||||
| 8 HOURS | 10 HOURS | 12 HOURS | |||||
| INOCULUM | INITIAL | FINAL | INITIAL | FINAL | INITIAL | FINAL | |
| 0% | 14.75a ± 020 | 14.18a ± 0.30 | 15.46e ± 1.40 | 14.84e ± 0.76 | 14.34j ± 0.61 | 15.45k ± 0.30 | |
| 1% | 13.31b ± 0.73 | 13.63 b ± 0.52 | 15.07f ± 1.44 | 14.97f ± 0.85 | 14.65l ± 0.61 | 14.90l ± 0.16 | |
| 5% | 12.83c ± 0.18 | 13.20c ± 0.49 | 13.96g ± 0.51 | 13.10g ± 0.42 | 13.90m ± 0.56 | 13.62m ± 0.89 | |
| 10% | 11.78d ± 1.53 | 12.10d ± 1.35 | 14.38h ± 0.35 | 14.20h ± 1.09 | 13.03n ± 0.35 | 12.99n ± 0.46 | |
The same letter indicates that there were no significant differences between initial and final stages.
A T-test was applied to test the differences between the beginning (Initial) and the end (Final) of the fermentation at 8, 10, and 12 hours. Results indicated no significant differences (α=0.05), except for the 12-hours fermentation and 0% of inoculum. According to 12, in cooked quinoa, sugars and starch rise to 17 g / 100 g; while in quinoa beverages, the values reach 3.4 g / 100 g. According to 29, these sugars consist of glucose 1.7 g, fructose 0.2 g, sucrose 2.9 g, and maltose 1.4 g (g / 100 g dry matter). Therefore, once the free sugars in quinoa and those released by enzymatic hydrolysis have been consumed, the probiotic microorganisms would begin to consume other carbon sources. Furthermore, since glucose, fructose, and other sugars are metabolized by lactic acid bacteria 30, starch will likely hydrolyze and generate glucose and fructose as products. Therefore, it can be assumed that microorganisms immediately consume the sugars released by their enzymatic action, which would explain why there is no significant variation in reducing sugars.
Soluble solids:
Table 3 shows the variation of soluble solids for all treatments. It was observed that the soluble solids vary in different patterns. For example, in the 8 and 10-hour treatments, the soluble solids decrease while they increase in the 12-hour treatments. Some controls showed variations too. As stated by 31, soluble solids represent an important answer in the study of a fermentation process. These parameter changes include the growth of lactic acid bacteria and the production of organic acids during fermentation.
Table 3 Soluble solids (°Brix ± 0.01) at different fermentation times and different inoculum concentrations
| Soluble solids (°Brix ± 0.1) | |||||||
| 8 HOURS | 10 HOURS | 12 HOURS | |||||
| INOCULUM | INITIAL | FINAL | INITIAL | FINAL | INITIAL | FINAL | |
| 0% | 10.00a ± 0.00 | 10.00a ± 0.00 | 10.00a ± 0.00 | 8.83f ± 0.29 | 9.33m ± 0.58 | 9.67m ± 0.29 | |
| 1% | 10.00a ± 0.00 | 9.17b ± 0.29 | 9.67g ± 0.29 | 8.83g ± 0.76 | 9.00n ± 0.00 | 9.50p ± 0.00 | |
| 5% | 9.00c ± 0.00 | 8.50d ± 0.00 | 8.50h ± 0.87 | 7.50h ± 0.50 | 8.17q ± 0.29 | 8.67q ± 0.29 | |
| 10% | 9.00c ± 0.00 | 8.50e ± 0.00 | 9.17i ± 0.29 | 7.17k ± 0.29 | 8.00r ± 0.00 | 8.17r ± 0.29 | |
The same letter indicates that there were no significant differences between initial and final stages.
The decreases in the 8 and 10-hour treatments coincide with the decreases in pH, suggesting that free sugars are being used to produce lactic acid. In the case of the 12-hour treatments, an increase was evident for all inoculum concentrations, including those of the control treatment. This result was similar to that obtained by Cañón Rodríguez 32, who stated that soluble solids increased in the final fermentation moments between 3.5 and 4.5 hours. Additionally, in research carried out by Lima et al. 31 in which they used Lactobacillus gasseri to produce probiotic food, soluble solids increased from 8 to 12 hours and decreased after 16 hours of fermentation.
Statistical analysis
The best treatment was determined based on the "Total Titrable acidity produced parameter. For this, a two-factor ANOVA was applied. For the effect of fermentation time, Fcal 27.63392 > F.tab 3.4; for the factor Concentration of probiotic microorganisms, F cal 119.3991 > F.tab 3.01; and for factor interaction, F.cal 3.105352 > F.tab 2.51. In all cases, Fcal>Ftab; therefore, both the fermentation time and the concentration of probiotic microorganisms significantly affected the samples. Afterward, a comparison test was made for the main and simple effects. The results indicated significant differences between the 8-hour and 10-hour fermentation but no significant differences between the 10-hour and 12-hour fermentation. Therefore, the fermentation of 10 hours was determined as the best treatment. The tests for simple effects concluded that, for a 10-hour fermentation, there were no significant differences between 10 % and 5 % of the concentration of probiotics, but there were between 5 % and 1 %. Using the 10 % concentration as a preventive measure was preferred to ensure the product’s safety since an initial population of 10 % has a greater probability of eliminating possible pathogens. In addition, it can reduce chemical preservative usage in food and can also have the potential to work in synergy with synthetic preservatives 33. Thus, together with potassium sorbate, it could extend the life of the product. Consequently, the best treatment was 10 hours of fermentation at a probiotic concentration of 10 %.
Proximal analysis
The formulated beverage, in mango flavor, prepared with 10 hours of fermentation and 10 % inoculum, was analyzed in La Molina Total Quality Laboratories. The probiotic quinoa beverage contains 84.6 kcal/100 g of sample for the caloric content. This energy was attributed to carob and honey, which provided sugars to the beverage. Regarding protein, its value is 1.4 g/100 g of beverage. It was understood that the value of the protein was about the dilution made (approximately one-tenth of the content in the quinoa grain).
Regarding carbohydrates, the probiotic quinoa beverage contains 19.3 g per 100 g, representing an advantage if it is intended to be consumed as an energy beverage. As for fat, the probiotic quinoa beverage contains 0.2 g/100 g. Crude fiber amounts to 0.1 g/100 g of sample.
Microbiological analysis
The results of the microbiological analysis are shown in Table 5.
Table 4 Results of the microbiological analysis.
| Microbiological analysis (CFU/mL) | ||
| At the end of fermentation | Two weeks later | |
| Total mesophilic aerobic bacteria | 1.15* 108 CFU/mL | 7.95x106CFU/mL |
| Total coliforms | < 3 CFU/mL | < 3 CFU/mL |
| Molds and yeasts | < 3 CFU/mL | < 3 CFU/mL |
| Lactobacillus spp. | 6.25* 107 CFU/mL | 3.9x106 CFU/mL |
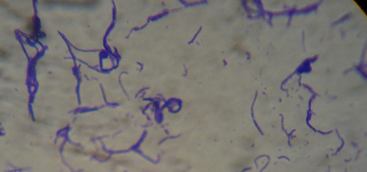
Considering that the inoculum was 10 % (2.53x106 CFU/mL), it was verified that the probiotic microorganisms have multiplied in the quinoa beverage. Analysis after two weeks confirms that the probiotic microorganisms were still present in the beverage, although in smaller quantities. This suggested that the beverage will continue to have the desired effect, even after two weeks of preparation. Gram stain results of a colony grown on MRS agar (Figure 2) revealed the presence of bacilli in chains (Streptobacilli).
At a magnification of 1000X, the image suggested that it would be the species Lactobacillus delbrueckii subsp. bulgaricus and Lactobacillus acidophilus, as these genera are gram-positive rods. The information provided by 19 stated that the probiotic microorganisms used include the genera mentioned above.
Discussion
The beverage in this research contained between 0.10 and 0.23 % w/w of lactic acid. This concentration was lower than that reported in the work of Díaz & Villa Fonseca 34, who elaborated a yogurt from yellow pitahaya, finding acidity values between 0.6 % w/w and 0.7 % p/p. Secondly, our results were similar to those found by Correa & Cuenca 23, who showed that around 24 hours of fermentation in some soybean beverages were required to reach adequate lactic acid levels (this is a pH between 4 and 5). This could be explained by considering that milk and other fermented dairy products provide probiotic matrices 35. On the other hand, quinoa -although a rich source of nutrients- has a different composition than milk. In this sense, organisms such as Lactobacillus delbrueckii subsp. bulgaricus, which not only ferments lactose, would be very suitable for vegetable substrates; instead, L. delbrueckii subsp. Lactis that have the enzyme β-galactosidase could only react in the presence of lactose 23.
On the other hand, even when the percentage of lactic acid was lower, the pH reached inferior values than those found in yogurt. This result was similar to Cuenca et al.36; although the production of lactic acid in a beverage fermented from soy was less than that of a beverage fermented from milk (yogurt), the pH reached the same values for both beverages. The reason would be the amount of protein in these foods since the abundant amino acids in milk proteins can act as buffer systems that regulate pH variations. Thus, with an increase in the concentration of H+ protons, the amino group of the protein accepts these protons 37.
The probiotic quinoa beverage provides more calories than other dairy probiotics considering the proximal analysis. It is 12 times more energetic than VitaBiosa, and about 500 times more than BioLaive yogurt and Kevita beverages 38. Regarding proteins, our product contained more protein than VitaBiosa and Kevita beverages but less protein than BioLaive yogurt 38. It should be considered that compared with cereal crops (rice, maize, and wheat), quinoa includes most of the essential amino acids, with a composition that is adequate to the adults’ requirements 39. Regarding carbohydrates, the probiotic quinoa beverage had a similar concentration of carbohydrates to that offered by some probiotic yogurts (BioLaive yogurt 15.5 g, VitaBiosa 1.5 g, and Kevita 3 g 38. Compared to other beverages, it had less fat than BioLaive yogurt, but higher fat than VitaBiosa and Kevita beverages 38. While BioLaive contains 2 g of fat per 100 mL of beverage, VitaBiosa and Kevita contain no fat, and the probiotic beverage contains 0.2 g. In this sense, the probiotic quinoa beverage was low in fat compared to existing beverages on the market. Considering that traditional yogurts do not have dietary fiber, using pseudocereals -instead of milk- to make beverages would be an alternative to improve the functionality of this type of food 23.
In a study by Arenas-Suescún et al. 40, lactic fermentation was carried out from milk with quinoa. They found a final count of 5.61x106 CFU /mL after 3.7 hours. The same author reports that the counts required for a commercial yogurt are around 107 CFU/mL. Likewise, according to Shori et. al.41, to ensure the health benefits of plant-based fermented dairy products, probiotics must meet a minimum level of probiotic bacteria between 106 and 107 CFU/mL until the date of expiration. In this research, the probiotic microorganisms in the beverage are in the order of 108 at the end of its preparation and in the order of 106 up to two weeks after it. This ensures that, when consumed, it will provide the expected beneficial effects on health.
Notably, lactic acid bacteria are beneficial because they can prevent the growth of harmful enteric pathogens, supply enzymes, eliminate toxic food elements in the intestine, promote immunomodulatory action, stimulate the immune system, and enhance the peristaltic action of the gastrointestinal tract 42.
Conclusions
Enzymatic hydrolysis with α-amylase allowed the hydrolysis of quinoa flour starch. Lactic acid fermentation with 10 % inoculum and 10 hours of fermentation was the most suitable treatment to obtain a probiotic quinoa beverage. The proximal analysis confirmed that the beverage has 84.6 kcal/100 g, 19.3 g carbohydrates/100 g, 0.2 g fat/100 g, and 1.4 g protein/100 g per sample. Lactobacillus spp. is present in the beverage at a concentration of 6.25* 107 CFU/mL at the end of fermentation and 3.9x106 CFU/mL two weeks later. Therefore, the consumption of the probiotic quinoa beverage, in the recommended doses, would allow making the most of the nutritional benefits of quinoa and the beneficial effects of the probiotic microorganisms, being able to prevent the appearance of chronic non-communicable diseases. The benefits of consuming probiotic microorganisms are, among many others: prevention of diarrhea, reduction in symptoms associated with inflammatory bowel disease, prevention of gastrointestinal cancers, alleviation of lactose intolerance, reduction in Helicobacter pylori infection, prevention of intestinal inflammatory disorders and regulation of metabolic disorders, such as serum lipids, cholesterol levels, blood pressure and glucose regulation 43.
It is recommended for future research to determine the percentage of survival of probiotic cultures over a more extended period, simulating shelf life. Likewise, it would be convenient to evaluate the sensory acceptance and physicochemical stability (pH, titratable acidity) after 21 days of storage at different temperatures, as recommended by 44. In addition, cofactors and prebiotics that could enhance the yield of lactic acid would be a field to explore since, in this investigation, values of only 0.2% w/w were reached as a maximum. Furthermore, the determination of organic acids, free amino acids, and antioxidant activity 45, the addition or absence of preservatives, and the behavior of the beverage without adding additives would complement the information on this functional quinoa beverage. Finally, the exploration of different genera of lactic bacteria that could improve the beverage’s quality would be important. Weissella confusa is recommended by Boukid set al.46) due to its capacity to improve viscosity and mouthfeel in fermented products.