1. Introduction
Worldwide, the growing interest in the consumption of fermented foods has been noted, exploring low-cost traditionally fermented products containing an important source of nutrients, probiotic microorganisms, and help to improve the health and well-being of consumers. For this reason, many countries seek to rescue traditional foods and their consumption by exploring their therapeutic properties through scientific research that supports the importance of their consumption and incorporation into the diet 1-4.
Latin America is home to many indigenous communities that feature a wide range of fermented foods as part of their cultural principles and are symbolically preserved within their natural wealth and legacy. Some authors state that at least 21 indigenous foods are part of the daily preparations and those have been preserved in different cultures by recipes transferred from generation to generation 5. For this reason, some foods are considered human culinary heritage that maintain nutritional contribution to the population in providing essential macro and micronutrients for humans at any stage of growth 5-7.
Thus, traditional fermented foods have been elaborated from different matrices of local agricultural products, such as: sugar cane (Saccharum officinarum), cassava (Manihot esculenta), rice (Oryza sativa), coffee (Coffea), cocoa (Theobroma cacao) or corn (Zea mais) 8. Corn is one of the most widely used cereals to produce different types of indigenous fermented foods given its properties as a good substrate for the growing of different beneficial microorganisms. Some examples of these foods include: chicha (alcoholic fermentation), masa añeja (lactic fermentation) and champús (mixed fermentation), among others 9-11.
Champús is a traditional beverage or dessert mainly consumed Colombia, Ecuador, and Peru 8,12. It has a characteristic sweet to sour taste with a low alcohol content, and it’s preparation have some variations depending on each population; it is generally made from cereals such as corn, wheat or a mixture of these, however, may contain additional ingredients like pineapple (Ananas comosus), lulo (Solanum quitoense), panela honey (sugar cane), spices such as cinnamon (Cinnamomum verum), cloves (Syzygium aromaticum), vanilla (Vanilla), and aromatic plants such as orange tree (Citrus X sinensis) or arrayán (Luma apiculata) (7, 9). Champús is made from raw corn kernels that go through a grinding process, water is added, and the corn is left to ferment at room temperature for a period of 24 to 48 h. At the end of the fermentation period, a second grinding process is carried out to obtain fine and homogeneous particles that are again left to ferment from 24 to 48 h. The resulting mixture is subjected to cooking, where ingredients such as spices and aromatic plants are added until the desired viscosity is reached. The name Champús comes from the Quechua "Chapusca" that means mixture, or "Chapuy" which means to stir or beat. Finally, the mixture is allowed to cool and, for consumption, panela honey and "mote" (from the Quechua: mut'i) are added 14.
In general, fermented corn preparations are characterized by low cost, easy making and the availability of raw materials at any time of the year 14. However, these products are produced following poorly controlled processes without appropriate preservation techniques. Fermented corn preparations are produced on a small scale and marketed locally. This is the reason why it is recommended to monitor their hygienic and sanitary quality to prevent the transmission of foodborne infections 12,15.
Therefore, the purpose of this research was to establish a baseline for the microbiological and physicochemical quality of champús traditionally made by indigenous populations of southwestern Colombia and to study the microbial communities occurring in these fermented products aiming to reappraise these traditional foods.
2. Materials and methods
2.1 Participation of producers and collection of samples of champús
The samples were provided from five artisanal producers of champús from the municipality of Córdoba (Nariño, Colombia), located in the extreme southwest of the country in the Andean region (0°51′12″N, 77°31′04″W). The producers agreed to participate voluntarily signing an informed consent form document.
Each producer made two batches of champús keeping their own methodologies during the process. During the elaboration of each batch, the environmental conditions of temperature and relative humidity (T and % RH) were measured and four samples were taken for microbiological and physicochemical analysis at different stages of the process (EP): EP0 (the mixture of corn in water before starting fermentation), EP1 (the mixture at an intermediate time of fermentation, 48 h), EP2 (the mixture after finishing fermentation, after reaching pH ˂4) and EPf (final product). The samples were collected aseptically in whirl pack bags and frozen at -18 ± 2 °C and then sent in less than 48 hours to the laboratory of the Universidad de Antioquia (Medellín) for analysis.
2.2 Microbiological evaluation of champús
Each end product of champús (EPf) was evaluated for microbiological criteria: Total mesophilic counts (ISO 4833-2:2013), total coliforms (TC) and E. coli (ISO 4832:20068), Staphylococcus aureus (ISO 6888-3:2003), Bacillus cereus (ISO 7932:2004), molds and yeasts (M&Y) (ISO 6611:2004), as well as the test for the absence or presence of Salmonella spp. (ISO 6579-1:2017). In addition to these tests, surface seeding was performed for the count of acetic acid bacteria (AAB) using WL agar (Scharlau - Spain). After the corresponding incubation, the colonies were counted and expressed as Log CFU/g, for subsequent statistical analysis.
2.3 Lactic acid bacteria and yeast isolation
The abundance of lactic acid bacteria (LAB) and yeasts was evaluated during all stages of the process (EP0, EP1, EP2 and EPf). Culture media were used for LAB : Man Rogosa Sharpe (MRS - Oxoid - Spain) and M17 supplemented with 0.5 % lactose (Oxoid - Spain) and yeast extract - glucose - lactose - peptone - meat (YGLPB) prepared according to the instructions of the Spanish Type Culture Collection (CECT, http://www.cect.org). All media were supplemented with 100 µg/mL cycloheximide, and the Petri dishes were incubated at 30 ± 2 °C for 48 h in microaerophilic conditions. Yeast abundance was determined according to ISO 6611:2004 methodology. Finally, colony counts were performed and expressed as Log CFU/g.
From the LAB colonies obtained, those with different morphotypes were randomly selected and isolated on the agar from which they were initially obtained; after incubation under the above-mentioned conditions, those isolates that met the basic characterization of negative catalase, negative oxidase and positive Gram staining were considered presumptive LAB and preserved at - 80°C in a suitable broth containing 20 % glycerol.
Molecular identification of LAB isolates was performed by amplification and sequencing of the 16S ribosomal gene using the primers 785F (3' GGA TTA GAT CCC TGG TA 5') and 907R (5' CCG TCA ATT CCT TTR AGT TT 3'). The 16S rRNA sequences were compared with references LAB from the NCBI GenBank using BLASTN algorithms (http://www.ncbi.nlm.nih.-gov/blast).
2.5 Physicochemical analysis of champú
The pH was measured in real time during the fermentation process, in at least one of the batches from each producer using a pH-meter manufactured by the Interfaculty Instrumentation Center of the Universidad de Antioquia. In addition, the samples collected at different times were assessed for titratable acidity, expressing the result as percentage of lactic acid (% w/v; ISO/TS 11869:2012).
The content of organic acids (lactic, acetic, and propionic), simple carbohydrates (glucose, fructose and maltose) and ethanol were determined by high performance liquid chromatography (HPLC) according to the methodology proposed by Valencia et al. 16. Soluble solids (°Brix) were measured by refractometry considering as a reference the Colombian Technical Standards (NTC 440:2015). CIEL*a*b* coordinates for color were evaluated on X-Rite SP-64 portable spectrophotometer with D65 illuminator and 2⁰ observer with attached specular and 4 mm observation window 17. In addition, to determine the percentage of crude protein (Volumetric - Kjeldahl), free amino acids, ash (ISO 5984:2002), moisture (NTC 2227) and the concentration of calcium, iron, zinc, phosphorus (UV-VIS spectrophotometric) a bromatological analysis was performed in the bromatological analysis laboratory at Universidad Nacional de Colombia (Medellín) (Regulatory document PRE-010 VOO2018) 18.
2.6 Sensory analysis
The samples that met the microbiological criteria were analyzed by quantitative descriptive analysis by five trained judges who evaluated the objective attributes of color, odor, flavor, and appearance, with a hedonic scale where excellent score = 10 and very poor score = 0.
2.7 Statistical analysis of data
The statistical difference between treatments was determined using the 95 % standard deviation limits. An analysis of variance (ANOVA) was performed using IBM SPSS version 25 and Statgraphics version 18 to compare the results of the counts among process stages and producers.
3. RESULTS
3.1 Microbiological evaluation of champús
A total of 40 samples were collected from the producers at different production times. The temperature and relative humidity during sampling were between 16.5 ± 2.56°C and 61.6 ± 9.14 %. The total coliforms (TC) were above the permitted limits (< 1 Log CFU/g), considering as a reference the microbiological the Colombian Technical Standards NTC 805:2005 f++or dairy products and fermented milks, NTC 3594:2014 for milling products: precooked corn flour for human consumption and the resolution 1407 of 2022 where microbiological criteria are established that must be met by food and beverages destined for human consumption and which include fermented beverages whose alcohol content is less than or equal to 2.5 %. The average count of the Log CFU for E. coli in some champús were above the permitted limits (< 1 Log CFU/g). For S. aureus all the champús met the established control limits for this microorganism (< 2 Log CFU/g). The mold and yeast (M&Y) count averaged 4.43 ± 2.45 Log CFU/g and the acetic acid bacteria (AAB) showed an average of 6.18 ± 2.37 Log CFU/g. None of the samples evaluated showed growth of B. cereus and Salmonella sp. (Figure 2).
3.2 Lactic acid bacteria and yeast isolation
The average LAB and yeast log CFU counts reveal the predominance of fermenting microorganisms during the process stages in which the samples were taken; these counts show greater variation for the initial and final time of the process (Figure 3). The average initial concentration of LAB was 5.1 ± 0.99 Log CFU/gand presented a significant increase (p ˂ 0.05) in EP1, reaching the maximum cell concentration for the batch (8.9 ± 0.42 Log CFU/g). these LAB from EP1 did not exhibit statistically significant differences with EP2 (p=0.068). In EPf, they showed an average of 6.6 ± 0.3 Log CFU/g.
On the other hand, the yeast counts show a greater dispersion of data in EP0 (˂1 and 4.07 Log CFU/g) and in EPf (4.22 ± 1.42 Log CFU/g), obtaining statistically significant differences between EP0 (p=0.000) compared toEP1, EP2 and EPf that show homogeneity among the counts (p=0.777).

Figure 3 Averages and standard deviation expressed in Log of CFU/g of lactic acid bacteria and yeasts during each stage for fermentation process of champús.
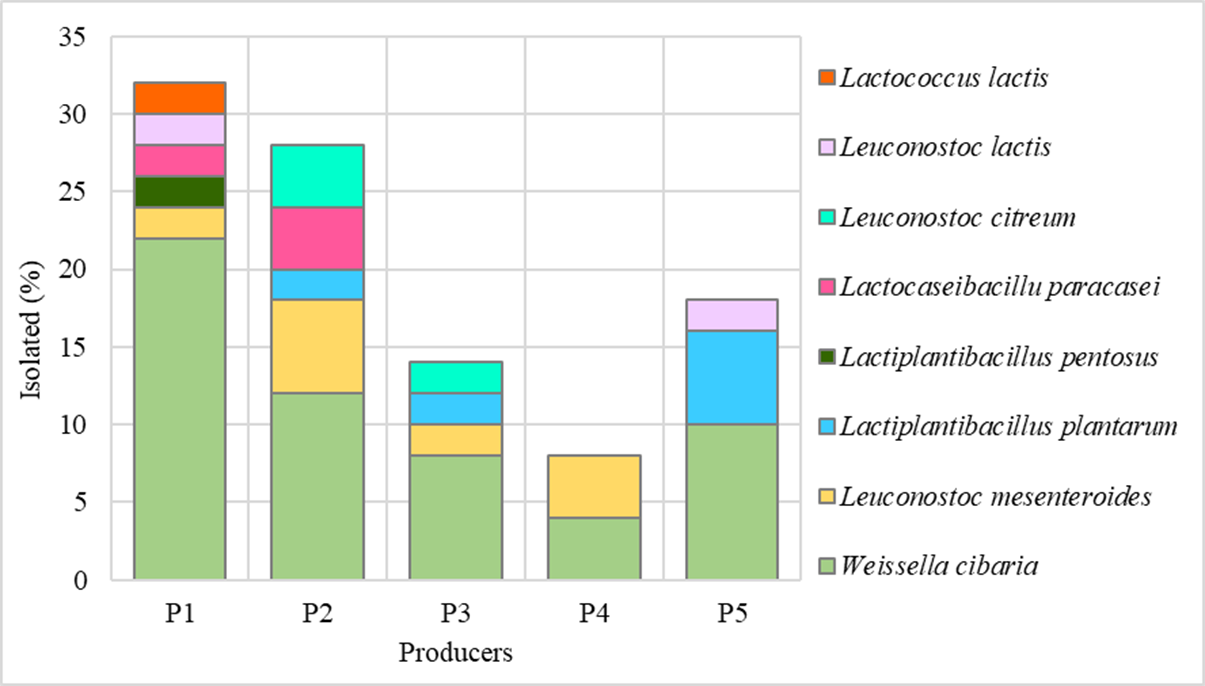
From the samples taken at the different stages of the process, a total of 171 isolates were obtained among the five producers; these isolates met the basic criteria characteristic for LAB presumptive colonies. The percentages of isolates per producer were as follows: P1 23.98 %, P2 21.64 %, P3 15.2 %, P4 16.37 % and P5 with 22.81 %. For their identification, 50 isolates with antagonistic activity against pathogens were selected (Figure 4) and complied with their safety characterization (hemolysis, collagenase, lecithinase, and biogenic amines, these data were not shown). A fragment of ~ 1000 pb of the 16S rDNA gene was obtained and BLASTN results revealed the presence of Weissella, Leuconostoc, Lactobacillus, and Lactococcus, with a percentage of identity from 99,2 - 100 % and with a higher prevalence of Weissella cibaria (56 %) and Leuconostoc mesenteroides (14 %).
3.3 Physicochemical analysis of champús
The values of pH and titratable acidity expressed as percentage of lactic acid (%LA) evaluated in different stages of the process are shown in Figure 5. Three homogeneous groups were observed during fermentation. The first group formed by the EP0 data with an average pH 6.02 ± 0.16, the second group by the EP1 data and the third homogeneous group was formed by EP2 and EPf, reaching an average pH 3.82 ± 0.25.

Figure 5 Averages and standard deviation of acidity expressed as percentage of g/mL of lactic acid and averages and standard deviation of pH during the different stages of the champús production process.
Regarding titratable acidity, no statistically significant differences were observed among the stages of the process (p = 0.203), although the EP0 data with an average acidity of 0.34 ± 0.3 % LA (g/mL) showed greater variation. In addition, although the pH barely varies between EP2 and EPf, the acidity tends to increase in the finished product, maintaining a high variation among samples.
The results of organic compounds measured on HPLC in EPf (Figure 6) confirmed the presence of fermentation metabolites. Lactic acid and ethanol had the highest concentration in the fermentation metabolite concentration test, followed by acetic acid and propionic acid, which was below the detection limit range in some test samples (< 0.05 g/L). The concentrations of these compounds showed a statistically significant difference between them (p < 0.05). In addition, a 2:1 ratio was observed between the means of lactic acid and acetic acid.

Figure 6 Concentration of metabolites of interest. Mean and standard deviation of ethanol, organic acids, and simple carbohydrates concentration expressed as (g/L).
Likewise, in ethanol production, a high variation was found among the data (from 2.8 to 27.58 g/L). Furthermore, the presence of glucose and maltose as a product of corn starch hydrolysis was also evidenced. The presence of fructose probably resulted from the addition of panela honey to the final product. All these sugars showed great variation in their concentrations.
The results of the bromatological analyses, mineral content, free amino acids, moisture percentage and soluble solids are presented in Table 1. The data obtained show that champús is a rich source of minerals and free amino acids.
| Composition | Units | Mean ± SD |
|---|---|---|
| Humidity | g/100g | 76.73 ± 5.10 |
| Soluble solids | ° Brix | 16.67 ± 3.90 |
| Free amino acids | mM | 0.68 ± 0.23 |
| Ashes | g/100g | 0.27 ± 0.07 |
| Phosphorus | mg/kg | 321.20 ± 111.52 |
| Calcium | mg/kg | 119. 20 ± 73.97 |
| Zinc | mg/kg | < 2.50 |
| Protein | g/100g | < 2.50 |
Regarding color, the ∆E (1.86 ± 1.56) shows that color differences are not perceptible by the observer (˂2). Brightness (L*) presented an average of 37 ± 2.70 with a predominance of yellow tones (b* = 11.62 ± 1.51 and a* = 1.43 ± 0.51).
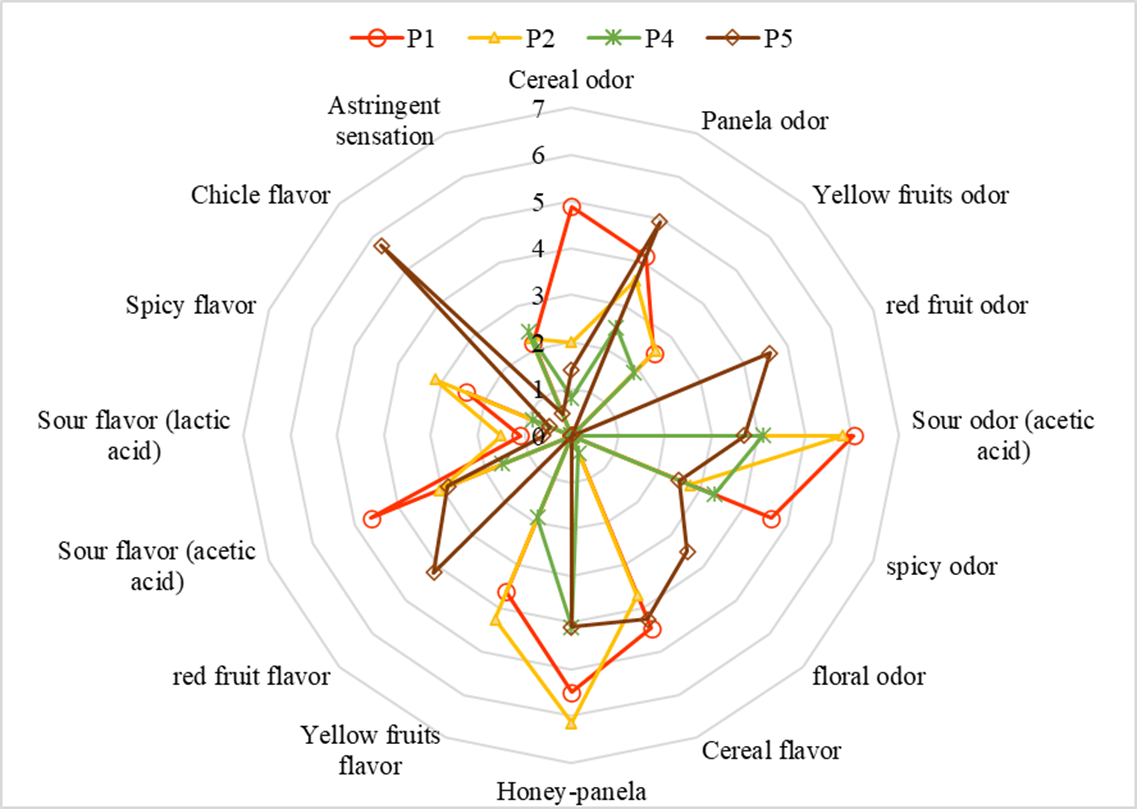
Finally, the average of the data obtained in the sensory profile for four producers is shown in Figure 7. It can be observed that the intensity of the descriptors was below 6.15. The panelists highlighted descriptors such as honeycomb, sour, acidic flavors, and panela honey. Another descriptor named was spicy odor. In addition, there is evidence of variation between producers due to changes in the manufacturing processes and the lack of standardization in the amounts of raw materials added.
4. Discussion
The results obtained in the microbiological criteria featured a high dispersion of the data, probably associated to the lack of standardization and contamination problems after the cooking process. This contamination also may be due to the deficiencies in the facilities (water used, utensils, wooden tables, or presence of insects) and the addition of ingredients that are not heat-treated, such as mote and panela honey. Fermented products with similar contaminations have been discussed by other authors in the artisanal production of champús 11,14 and fermented rice beverages 16,19,20. The presence of microbiological safety criteria such as S. aureus and E. coli in a ready-to-eat product indicates that this food could be a vehicle for the transmission of foodborne toxi-infections. Consequently the presence of these microbial groups is undesirable and should be controlled 21.
It should be noted that there are no established microbiological criteria for fermented foods such as champús; this regulatory deficiency has been reported by other authors who have evaluated traditional fermented foods 8,19,22-24.
Regarding AABs, they are usually found in environments rich in carbohydrates, polyols or ethanol. They produce acetic acid which could explain the evidenced concentrations of this microbial group. Still, the role of these microorganisms is unclear in terms of functionality and microbiological interactions 25, but acid production reduces the presence of acid-sensitive coliforms and enterobacteria. In fact, producers with higher AAB counts are the ones that presented lower pathogen counts.
On the other hand, LAB presented similar counts compared to those found in studies on rice 19 and corn-based 26,27 fermented foods and beverages. Variations in the results could be equally related to the use of utensils, water sources and others. These microorganisms are part of food systems and are of great importance in natural fermentation processes, since their survival capacity is related to the ease of metabolizing a wide variety of carbohydrates and adapting to stress conditions (e.g., low pH) 28.
In addition, it is observed that yeasts like LAB play an important role in the fermentation of champús since they can be found as autochthonous microbiota in corn 19. Thus, Osorio et al., in their research identified wild yeasts in champús,obtaining 235 isolates 22.This indicate that in fermentations made with cereals such as corn, rice, or wheat, yeasts are also part of the diverse microbiota of these foods, as mentioned by other authors 10,29-31.
The different treatments of the champús process contribute to the prevalence of some microbial species and the interactions favoring the coexistence of these species. Several studies have reported the presence of some microbial genera in fermented corn-based products, among which the more common genera are Lactobacillus (L. plantarum and L. fermentum), Pediococcus, Leuconostoc (Lc. lactis, Lc. mesenterioides, Lc. citreum), Enterococcus, Lactococcus and Weissella (W. cibaria and W. confusa) as dominant microorganisms in fermented foods from Latin America and Africa 15,21,26,27. Some of these species were isolated from the final products in this study.
Indeed W. cibaria is a microorganism found in a wide variety of habitats, like soil and fresh vegetables and its presence is associated with the milling of corn kernels 20,28. Its probiotic characteristics together with those of Lc citreum were evaluated in a fermented food from Mexico where the ability of W. cibaria to inhibit the growth of pathogens such as S. aureus, Salmonella enterica biovar Typhimurium and Listeria Monocytogenes was identified 35. This ability is explained by the production of antimicrobial compounds such as bacteriocins and short-chain organic acids (formic, propionic and butyric acids) 36,37.
Leuconostoc is part of the secondary (non-fermentative) microbiota in fermented vegetable foods 20. Additionally Lc citreum is remarkable for its high production of short-chain fatty acids such as propionate and butyrate 29,35,38.
Other studies highlight that LAB belonging to the genera Leuconostoc and Weissella are exopolysaccharide producers, although this characteristic is strain dependent.This metabolite favors some physicochemical characteristics of the product such as viscosity and texture. Finally, L. plantarum, L. lactis and W. cibaria strains have been used as starters for beverage fermentation 39-41.
The pH results obtained in this research is similar to those in previous reports 4,20,31,32,34. Additionally, the acidity presented in the EP0 could be related to the physicochemical characteristics of corn, which may contain a titratable acidity between 0.18 to 0.3 % depending on the corn variety. This acidity could be due to the organic acids present, and these are associated with the soil conditions during cultivation 43. Although the other stages of the process varied, they showed tendencies to increase the acidity concentration probably due to the degradation of the substrates and the production of organic acids resulting from the metabolism of the microorganisms present. Furthermore, the variation in these stages could be related to the presence of different types of microorganisms (Figure 3) 19,30. Moreover, these differences could also be due to the partial dissociation of weak acids that may be in fermented products 44.
Otherwise, the process of making champús is fulfilled in several stages, beginning with the reduction of the particle size of the corn grains which are then hydrated for 24 h. Later fermentation begins with physicochemical changes experienced by champús. When the grains are soft, they undergo a second grinding where water is added. Finally, grinded corn is boiled for 2.5 ± 0.5 h with a ratio of water and grinded corn of 2:1 until complete starch jellification is reached. These steps lead to physical and chemical changes during fermentation, in which both the washing and the water addition decrease the concentration of the produced acids and cause the longer acidification steps. This prevents the increase in percentage acidity and, at the same time act as selective agents of the microbiota responsible for fermentation and modification of the substrate 21,34.
The proportions of the produced acid possibly provide to the product different sensory characteristics, e.g., lactic acid is sensed with a "mild acid" taste while acetic acid is closely related to unpleasant sensory characteristics such as sour tastes and odors 28,45. On the other hand, propionic acid production has been described as a final product produced by the metabolism of some microorganisms and it can inhibit growth and microbial activity of molds and some bacteria. Propionic acid is therefore used as a food preservative 37,46,47.
The presence of ethanol is closely related to the metabolism of yeasts and the variations in the percentage of ethanol could be related to the elevated counts of yeasts presented in the final products (Figure 2 and 3), since this group of microorganisms hydrolyzes starch into sugars for use in the ethanol production 7,25,41. This sugar consumption is associated with the lower glucose concentration found in some of the champús samples. Additionally heterofermentative LAB (L. plantarum, L. pentosus, L. paracasei and Weissella among others) are also present and they could metabolize glucose by the phosphoketolase metabolic pathway and produce lactic acid, CO2 and ethanol 22,42.
The bromatological results of the champús are related to the composition of corn, which contains minerals and vitamins that can change in concentration rate according to its genetic variety and the type of soil where it was planted. For example, the iron content was only evident in some of the champús samples (6 ± 1.4 mg/kg) made with white corn, providing 60 % of the daily food requirements in children between 4 and 8 years old 50,51. On the other hand, the iron content of those made with yellow corn was below the detection limit (<5 mg/kg). Corn also stands out for being a good source of carbohydrates and digestible fiber, and although it is not a good source of protein, it provides free amino acids (FAA), with leucine being the most abundant according to studies and the most important in children between 9 and 13 years old 35,43,44.
It should be mentioned that within the LAB metabolism, the fermentation process induces the hydrolysis of proteins and peptides due to proteolytic enzymes transforming raw materials, increasing the concentration of FAA in the food through the metabolic pathway of amino acid metabolism 54, producing fatty acids. However, α-carboxylic metabolites involved in LAB metabolism, act as antimicrobial agents and confer specific organoleptic characteristics and biogenic amines that may be beneficial for LAB survival but are toxic for to human consumption 54-56.
Some studies have shown an increase in FAA in fermented foods compared to their controls. Ahmads et. al. evaluated fermented milks noting that the FAAs produced provide complexity towards the sensory attributes and could impart the kokumi flavor of the final product. Finally, amino acid metabolism involving glutamine, glutamic acid, and arginine helps LAB adapt to the acidic environment 54,55,57.
Additionally, champús has minerals such as calcium providing 10 % of the daily nutritional requirements for an adult 51,58 and despite being in a low concentration, its consumption is of great importance. Especially, when a challenge has currently been evidenced in food safety by finding deficiencies in the consumption of this mineral, hence champús may promote the intake of calcium in the diet 59-63. Phosphorus provides up to 46 % of the daily nutritional requirements of phosphorus needed by children over 4 years of age and adults 51 ,64) , and champús is a rich source of this mineral.
The chromatic coordinates of champús are also associated with the phytochemical properties of corn. Yellow corn contains a relatively larger number of carotenoids, anthocyanins and phenolic compounds than white corn 17, 65, 66). Besides, panela honey is added to the product at the time of consumption. Panela honey is obtained by the Maillard reaction that takes place when Panela (a sugar cane product) is submitted to high temperatures resulting in a brown and carbohydrate-rich syrup. When added, it contributes to the flavor and color of the champús (67) .
In relation to sensory characteristics, these products have strong characteristic aromas and flavors that can sometimes be unpleasant for consumers. Many of these descriptors are related to metabolites formed during fermentation processes and included in raw materials used for their preparation, as well as the amounts used, the type of microorganisms found in greater abundance in the matrices and the processing and fermentation times.
Finally, the elaboration of handmade fermented foods like champús is susceptible to improvement without affecting tradition since some of these products are elaborated with poorly controlled processes without adequate technology.
5. Conclusions
Some of the products do not meet the microbiological quality criteria and there is a high variability in the physicochemical results. all this is related to the lack of standardization of the production processes and opens doors for further analysis where the combination of independent and dependent culture techniques may be used for a detailed and reliable investigation in difficult isolation microbial communities present during each of the fermentation processes of the champús.
Fermented foods despite having quality problems are a good source of beneficial microorganisms and nutrients that could become part of the regular diet of consumers.
These studies provide relevant information that can be used by the competent authorities to monitor and to improve production processes of champús, as well as to establish specific standards that include appropriate criteria for acceptance or rejection, ensuring the safety of this handmade fermented food. Finally, these studies also provide information that points to the isolation of microorganisms as defined starter cultures offering aggregated value to healthcare contribution.